Avicenna Journal of Pharmaceutical Research. :74-81.
doi: 10.34172/ajpr.2023.1058
Original Article
In Vivo Coagulation Effects of Thymus vulgaris Leaves Extract in Mice
Zahra Sadat Mashkani 1  , Jafar Vatandoost 1, *
, Jafar Vatandoost 1, *  , Toktam Hajjar 1, Behnam Mahdavi 2
, Toktam Hajjar 1, Behnam Mahdavi 2
Author information:
1Department of Biology, Hakim Sabzevari University, Sabzevar, Iran
2Department of Chemistry, Hakim Sabzevari University, Sabzevar, Iran
Abstract
Background: In addition to current therapies, herbal medicine is still used as an effective method for the treatment of some diseases such as bleeding disorders. Thymus vulgaris has been employed as traditional remedy in the treatment of bleeding disorders, although its clinical effects have not been investigated yet. Accordingly, this study evaluated the effects of the T. vulgaris extract on coagulation parameters, including bleeding time (BT), clotting time (CT), prothrombin time (PT), activated partial thromboplastin time (aPTT), and the number of platelets (PLT).
Methods: Forty male mice were randomly divided into 5 groups (n=8). The treated groups were administered with 100, 200, or 300 mg/kg/daily of the hydroalcoholic extract, as well as negative and positive control groups. Blood samples were taken from animals on the 13th and 14th day after treatment, and then coagulation indices were determined finally.
Results: The results demonstrated a significant reduction in the BT and CT tests while a significant increase in PT, aPTT, and platelet numbers. Phenolic and flavonoid compounds in the hydro-alcoholic extract of T. vulgaris are the most affecting compounds confirmed by gas chromatography-mass spectrometry and phytochemical experiments.
Conclusion: In general, the results indicated the coagulation effect of the T. vulgaris extract through primary homeostasis and a common pathway of secondary hemostasis.
Keywords: Thymus vulgaris, Primary hemostasis, Blood coagulation
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Mashkani Z, Vatandoost J, Hajjar T, Mahdavi B. In vivo coagulation effects of Thymus vulgaris leaves extract in mice. Avicenna J Pharm Res. 2022; 3(2):74-81. doi:10.34172/ajpr.1058
Introduction
Homeostasis including procoagulant, anticoagulant, and fibrinolysis system (1) may damage during coagulation disorders (2). Despite great advances in current therapies for bleeding disorders, including replacement therapy, gene therapy, and liver transplantation, they have low efficacy and significant adverse complications. Medicinal plants have played an important role in the treatment of diseases, and the use of their effective compounds is a new method for the treatment of bleeding disorders (3,4).
Thymus vulgaris of the Lamiaceae family grows in southern Europe, including Spain, Italy, Greece, Portugal, and France (5). Medicinal uses of this plant are related to various compounds (6). The chemical composition of T. vulgaris includes polyphenols and flavonoids (5), carvacrol, para-cymene, borneol, and gamma-terpinene (7), naringin and luteolin (8), tannins, as well as resins, terpenes, saponins, thymol, beta caryophyllene, and paracetamol (9). It has been demonstrated that tannins and flavonoids have a positive effect on the blood clotting process (5,10,11). It was also reported that alkaloids extracted from T. vulgaris had a positive effect on the reduction of bleeding time (BT) (12). The coagulation effects of the T. satureioides extract, a family of T. vulgaris, have been proven as well. The effect of the T. vulgaris extract on thrombosis and hemostasis was also demonstrated in vitro (13). Therefore, the in vivo assay of T. vulgaris as one of the effective coagulation plants will be useful.
Materials and Methods
Extraction
The fresh leaves of T. vulgaris were prepared from farms around Sabzevar, Iran. After identification, these leaves were washed under running water, shade dried, powdered into small pieces, mixed with 70% ethanol, and finally placed on a shaker for 48 hours at 100 rpm. The extract was filtered and concentrated at 55°C by rotary evaporation. The extract was then placed in a drying oven at 40°C to drive off the ethanol and water excess. The dried extract was kept at 4°C and used for further study (14).
Animals and Treatment Groups
Forty male NMRI mice (25-30 g, 6-8 weeks old) were purchased from the Animal Center, Royan Karaj, Iran. They were housed under normal laboratory conditions (21 ± 2°C, 12/12-hour light/dark cycle) with free access to standard rodent chow and water. The animals were adapted for 2 weeks prior to the experiment. Based on the statistical analysis with G*Power software, the instruction of Hakim Sabzevari University’s animal ethics committee as well as similar articles (15-17), three groups (n = 8) were designed for dosage of 100, 200, and 300 mg/kg/d. Negative and positive control groups (n = 8) were orally administered with 0.3 cc distilled water and 1200 mg/kg/d tranexamic acid, respectively.
Prothrombin Time
Each group was administered by gavage for 14 days and after this period, the mice were anesthetized using a ketamine-xylazine. Blood samples taken from the heart of the mice were collected into 3.2% sodium citrate (1 mL of citrate: 9 mL of blood) and centrifuged at 2500 rpm for 15 minutes. For the PTassay, 100 μL citrated plasma and 100 μL of warmed thromboplastin solution (Thermo Fisher) were mixed and incubated for 7 seconds at 37°C, and BT (the formation of the first white fibrin filaments) was recorded accordingly. Considering that thromboplastin reagents produced by different companies have different international sensitivity indexes, the international normalized ratio (INR) was used to compare the results of the prothrombin time (PT) test in different laboratories and eliminate the interference between the sensitivities of different reagents. The INR represents the ratio of experimented PT divided by a control PT obtained by using the international reference thromboplastin reagent developed by the World Health Organization (18,19).
Activated Partial Thromboplastin Time
For the activated partial thromboplastin time (aPPT) assay, 100 μL of the prewarmed aPTT reagent (Thermo Fisher) was mixed with 100 μL of citrated plasma and incubated for 3 minutes at 37℃. The clotting time (CT) was recorded after adding 100 μL of a prewarmed CaCl2 solution (1 mM) to the mixture.
Bleeding Time
BT was measured based on the Dejana method with some modifications (20) on the 13th day. BT was assessed by amputating 2 mm of the tail tip, and the issuing blood was carefully blotted every 15 seconds using the rough side of a filter paper. When no further blood appeared on the filter paper, the number of bloodstains on the filter paper was counted, and BT (seconds) was calculated by multiplying the total number of blood stains by 15.
Clotting Time
The CT was evaluated based on the method suggested by Li and White (21). On the 13th day, the tail tip was punctured with a scalpel, and a drop of blood from the supraorbital vein was collected on a glass slide. The CT was recorded between blood collection and fibrin formation (21).
Platelet Count
Platelet count (PC) was performed manually. On the 13th day, each tail tip was punctured, and a drop of blood was collected and smeared on a glass slide. The dried blood smear was incubated with methanol for 3 minutes and stained with Giemsa dye for 15 minutes. After washing and drying at room temperature, platelets were counted from 10 scopes and their mean was recorded as well (22).
Measurement of Total Phenolic and Flavonoid Compounds
The total phenolic content (TPC) of the plant extract was measured using a spectrophotometric method with some modifications (23). The reaction mixture was prepared by mixing 0.5 mL of methanolic solution of the extract (1 mg/mL), 0.5 mL of 10% Folin-Ciocalteu’s reagent in water, and 2 mL of NaHCO3 (10%). Blank was also concomitantly prepared, and the samples were incubated in a dark space for 2 hours at room temperature. The absorbance was determined using a spectrophotometer at 765 nm. The samples were prepared in triplicate for each analysis, and the mean value of absorbance was obtained accordingly. The same procedure was repeated for the standard solution of gallic acid, and the calibration line was drawn from it. Based on the measured absorbance, the TPC was calculated using a calibration curve and expressed in terms of gallic acid equivalent (mg of GAE/g of the dry extract) (23).
The TFC of the T. vulgaris extract was also determined using the aluminum chloride method (23) with some modifications. The sample contained 1 mL of the methanol solution of the extract at the concentration of 1 mg/mL and 1 mL of 2% AlCl3 solution dissolved in methanol. The samples were incubated for 30 minutes at room temperature. The absorbance was computed using a spectrophotometer at 415 nm. The samples were prepared in triplicate for each analysis and the mean value of absorbance was obtained accordingly. The same procedure was repeated for the standard solution of rutin, and the calibration curve was drawn accordingly. Based on the measured absorbance, the TFC was calculated using the rutin calibration curve. The TFC of the T. vulgaris extract was expressed in terms of rutin equivalent (mg of RUE/g of the dry extract) (24).
Gas Chromatography-Mass Spectrometry Analysis
To identify individual components, a hexane solution of the T. vulgaris extract was subjected to analysis on an Agilent gas chromatography-mass spectrometry (GC-MS) system (Agilent GC 6890A equipped with an Agilent 5973 mass detector) using the ZB-5ms capillary column (30.0 m × 0.25 mm i.d., 0.25 µm film thickness, from Zebron). The employed oven temperature programming is explained as follows:
Accordingly, its initial temperature was adjusted at 50°C for 5 minutes and then raised to 150°C by a ramp of 5°C/min. The oven was set at this temperature for 10 minutes. Finally, the open temperature was again raised to the final temperature of 260°C using a ramp of 5°C/min and held at this pressure for 20 minutes. The injector temperature was 260 °C. Helium was used as a carrier gas with a flow rate of 1.0 mL/minute. The samples were injected in the splitless mode. The adjusted operational parameters for the mass detector were the ionization voltage of 70 eV and ion source temperature of 200°C over a mass range of 500-500 amu. The peak area was determined using MSD ChemStation from Agilent Technology. A library search was performed for all the peaks using the NIST Mass Spectral Library software. The homologous saturated hydrocarbon standards (C8-C20 and C21-C40) were analyzed using the same column and conditions to calculate the retention indexes (RI) of compounds (25,26). The detection of compounds was based on a comparison of the measured RI and mass spectral patterns with those available in the literature. All the peaks with a match quality of ≥ 90% were considered and their names were specified accordingly.
Statistical Analysis
The analysis of variance was used to analyze the difference between the means of more than two groups followed by Tukey’s multiple, Games-Howell comparison test, and transformation test. The statistical significance level was accepted for P <0.05.
Results
Prothrombin Time and Partial Thromboplastin Time
The increased coagulation time was observed in the PT test of treated groups with the T. vulgaris extract in a dose-dependent manner up to 16.3 seconds for a dosage of 300 mg/kg/d. The significance analysis of data demonstrated that this time in the treated groups has significantly increased by 1.2 folds compared to the control (P < 0.05, Figure 1a). The same results were obtained for the aPTT test and in a dose-dependent manner, the coagulation time was significantly increased from 16.6 up to 30.3 seconds (1.8 folds) for a dosage of 300 mg/kg/d (P < 0.05, Figure 1b).
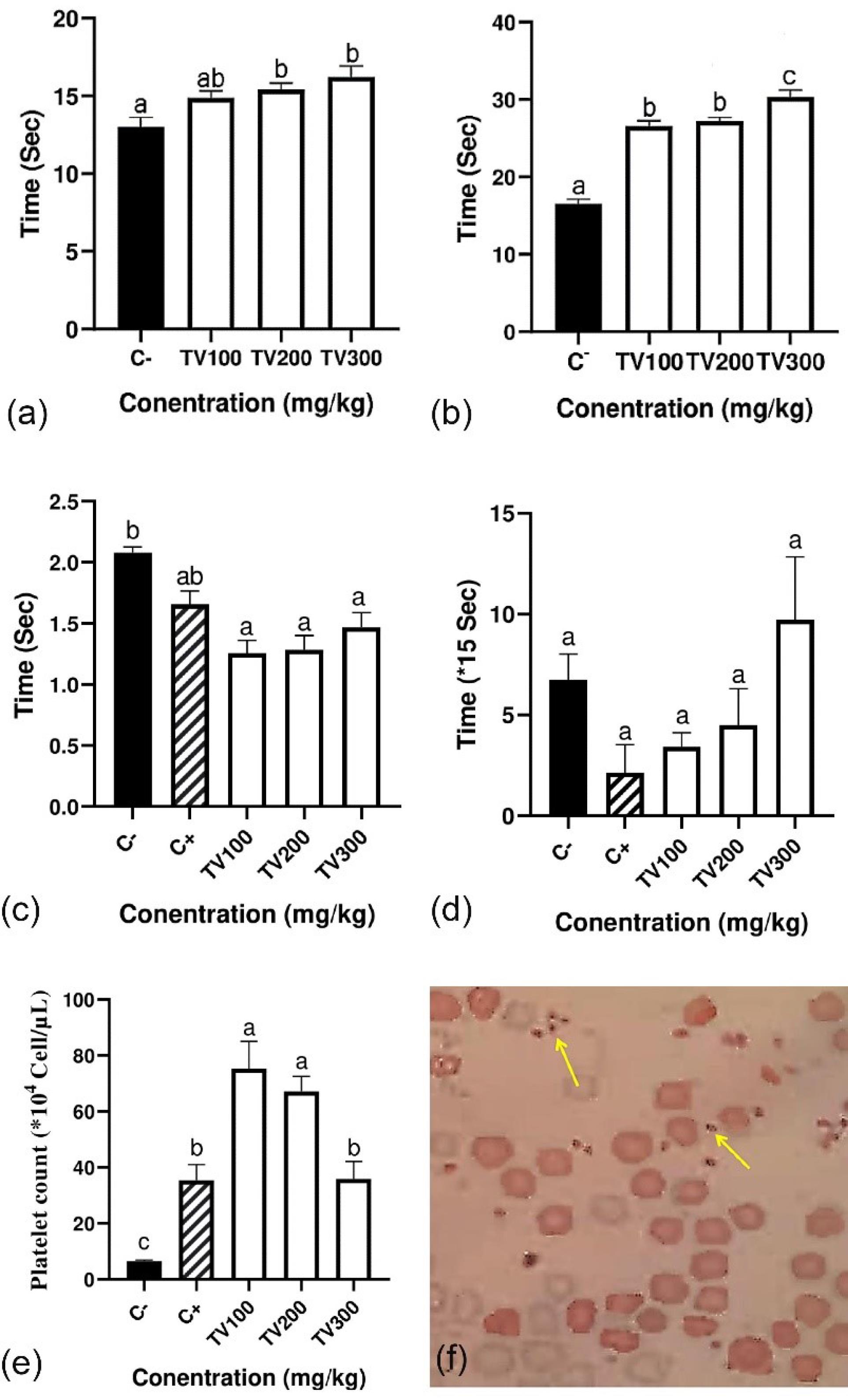
Figure 1.
In Vivo Coagulant Effect of the Thymus vulgaris Hydro-alcoholic Extract on Blood Coagulation Parameters: (a) PT, (b) aPTT, (c) CT, (d) BT, (e) Platelet Number, and (f) Microscopy Image of Platelet. Note. PT: Prothrombin time; aPTT: Activated partial thromboplastin time; CT: Clotting time; BT: Bleeding time; SD: Standard deviation. The data are the means ± SD of three individual experiments, and the significance of the data was shown with different letters
.
In Vivo Coagulant Effect of the Thymus vulgaris Hydro-alcoholic Extract on Blood Coagulation Parameters: (a) PT, (b) aPTT, (c) CT, (d) BT, (e) Platelet Number, and (f) Microscopy Image of Platelet. Note. PT: Prothrombin time; aPTT: Activated partial thromboplastin time; CT: Clotting time; BT: Bleeding time; SD: Standard deviation. The data are the means ± SD of three individual experiments, and the significance of the data was shown with different letters
Bleeding Time and Clotting Time
Although prolonged CT was observed in a dose-dependent manner, the findings indicated that the CT in all groups, the same as the positive control, was less than the negative control (Figure 1c). The effect of the ethanolic extract of T. vulgaris (Figure 1d) was accompanied by a non-significant increase in BT.
Platelet Count Test
The results of PC showed that the mean platelet number in negative control mice is 6.5 × 104 cell/µL, while this average in treated mice is 75, 67, and 35 × 104 cell/µL for TV100, TV200, and TV300, respectively which has increased significantly (P < 0.05, Figure 1e). Although a reduction was seen in treated groups in a dose-dependent manner, there was a significant increase of up to 11.5 fold compared with the control.
Analysis of Extract Compounds
The qualitative determination of different biologically active compounds from the T. vulgaris extract using GC-MS revealed 8 different compounds, in which phytol (30%), 2,6-dimethoxyphenol (23%), and thymol (19%), were the main compounds of T. vulgaris (Table 1).Moreover, the total phenolic content and the total flavonoid content of the extract were 1134 mg gallic acid/g and 0.08 mg Rutin/g of the sample in dry weight (mg/g), respectively.
Table 1.
Some GC-MS Identified Phytochemical Components of the Hydroalcoholic of Thymus vulgaris Leaf Extract
|
Compound Name
|
Area %
|
Compound Type
|
Structure
|
| Phytol |
30.18 |
Diterpene alcohol/diterpenoid |
|
| 2,6-Dimethoxyphenol |
23.57 |
Phenol |
|
| Thymol |
18.93 |
Terpenoids |
|
| 1-dienylcyclohexane |
7.4 |
Sulfur heterocycle |
|
| 2',4'-Dimethyloxanilic acid N'-veratrylidenehydrazide |
6.75 |
Alpha amino acid |
|
| 3,4-Diethylphenol |
4.84 |
Phenol |
|
| D,alpha.Tocopherol |
4.44 |
Phenols |
|
| α-Ionone |
3.89 |
Methyl ketones/noreisoprenoids |
|
Note. GC-MS, gas chromatography-mass spectrometry.
Discussion
In addition to current therapies for bleeding disorders such as replacement therapy, gene therapy, and liver transplantation, herbal medicines can be among the alternative methods. Although T. vulgaris has been used as a traditional remedy in the treatment of bleeding disorders, its clinical effects have not been investigated yet. Considering probabilistic coagulation compounds in T. vulgaris, an animal study was performed to evaluate the effect of its hydroalcoholic extract on mice. In line with in vitro experiments (27), our findings represented increasing the dose-dependent effect of the hydroalcoholic extract of T. vulgaris on the extrinsic coagulation pathway as prolonged coagulation time was observed in the PT test. Phenolic and flavonoid compounds, which were confirmed in quality tests, are possible factors affecting the extrinsic coagulation pathway (28). Thymol as an identified flavonoid compound in T. vulgaris (Table 1) can increase blood CT by reducing triglycerides (29) and subsequently reducing FVII activity (30). This mechanism was also demonstrated for the other flavonoid compounds such as Carvacrols (29,31) and Saponins (32,.(33Moreover, a decrease of triglycerides in the plasma serum has been reported in rats treated with T. vulgaris (34) or the Satureja hortensis oil extract (35). Another identified compound in the T. vulgaris extract, α-Ionone as a vitamin A precursor (36,37) can be another possible factor in the increase of PT since an increase in PT has been observed in chickens with vitamin A diet (38). Rosmarinic acid and hesperidin identified by other researchers (30,39,40) in the T. vulgaris extract indicated that they are the possible causes of increased PT (41).
The aPTT test reflecting intrinsic pathway function had a significant increase in the treated group compared to the control group (P < 0.05). These results are in line with in vitro results, showing that T. vulgaris extracts increase aPTT (27). It is supposed that some flavonoids and phenolic acids cause an increase in antithrombin III (ATIII) synthesis. ATIII is an activated form of protein C, leading to an increase in aPTT by the proteolytic cleavage of FVIIIa and FVa 82, 42, and 32. Polysaccharide compounds in T. vulgaris are the other possible factors in the increase of aPTT. The negatively charged polysaccharide and polyphenol-polysaccharide compounds can increase aPTT by increasing ATIII activity and inhibiting FVIII, FIX, and FXI activities (42). Polysaccharides can also inhibit the thrombin and internal coagulation pathway by the cofactor heparin II (43). It was shown that polysaccharide compounds extracted from Undaria pinnatifida (44), Codium Fragile (45), Porana volubilis (43), Camellia sinensis (46), Rosaceae, and Asteraceae family plants (47) prolong aPTT.
Platelet levels in T. vulgaris treatment groups increased significantly compared to the control group (P < 0.05). Thrombopoietin (TPO) is the main regulator of platelet production which is synthesized in the liver (48). This hormone binds to its receptor on the surface of platelets and megakaryocytes, increasing the TPO level and stimulating platelet production (48). Quercetin, which was previously identified by other researchers in the T. vulgaris extract, is the possible cause of increased platelet number through TPO effecting (48). In fact, quercetin increases TPO mRNA expression in bone marrow stromal cells (49).
It was also shown that polysaccharides in T. vulgaris increase platelets by affecting the runt-related transcription factor 1 (RUNX-1) and stem cell factor (SCF) genes. RUNX-1 is a transcription factor that induces megakaryocyte maturation, resulting in increased platelet production (50). The SCF gene is a stem cell and blood cytokine factor that causes megakaryocytes to mature (51).
The CT test reflects the function of a common and intrinsic pathway and platelet aggregation (52). The coagulation time in the CT test in the T. vulgaris treatment group was significantly reduced compared with the control (P < 0.05). It is supposed that beta-carotene in T. vulgaris increases iron and subsequently the number of red blood cells (RBCs), causing high hematocrit and activating platelet aggregation (53). RBCs are effective in inducing platelet aggregation by releasing a significant portion of their adenosine diphosphate (54). Released adenosine diphosphate from RBCs has a 60% and 28% contribution to the reduction of individual platelets and adhesion of platelets, respectively (54). It has been reported that alkaloids can reduce blood CT by inducing epinephrine (adrenaline) secretion (55), increasing the Factor V (FV) amount (56)
Linoleic acid in the T. vulgaris extract is another possible factor in reducing CT. In fact, linoleic acid by the desaturase enzyme produces arachidonic acid (57) that is converted to prostaglandin H2 by the cyclooxygenase enzyme. The conversion of prostaglandin to thromboxane α2 by thromboxane synthetase in platelets causes platelet aggregation and vasoconstriction (58). Thromboxane α2 synthesis can also be induced by tannins in the T. vulgaris extract. The inhibition of thromboxane α2 by quercetin and resveratrol reduces platelet aggregation (58). The gallic acid in the T. vulgaris extract in interaction with resveratrol and quercetin can inhibit their inhibitory effects on thromboxane α2, increasing platelet aggregation and CT (59). Polyphenols can stabilize low-density lipoprotein (LDL) through interaction with apoprotein B (60). LDL stabilization increases the possibility of OxLDL formation which can activate platelets through surface receptors and increase platelet aggregation (61).
The BT test, which is related to the number of platelets and vasoconstriction, is one of the most common tests for the identification of primary homeostasis disorders (62). This test indicates the formation of plaque hemostasis, which depends on sufficient platelet number and adhesion and reduced blood fluidity (63). The BT was reduced in the treatment group (except for TV300) with T. vulgaris compared to the control. It is supposed that the inhibition of vasodilators such as nitric oxide by T. vulgaris compounds can reduce BT. T. vulgaris compounds such as caffeic acid (64), retinoic acid (65), vitamin A (66), linalool (67), and carvacrol (68,69,70) reduce the production of nitric oxide in macrophages and result in vasoconstriction, platelet aggregation, and bleeding prevention.
Conclusion
According to CT, BT, and platelet tests, treatment with the T. vulgaris extract seems to be effective through the primary and common pathway of secondary hemostasis. In contrast, the T. vulgaris extract had prolonged effects on the intrinsic (aPPT) and extrinsic (PT) pathways of secondary hemostasis. Although the effect of the T. vulgaris extract on hemostasis tests is dose-dependent, TV100 is the best dosage for affecting the primary and common pathway of secondary hemostasis (CT, BT, and platelet number) without a significant impact on the intrinsic (aPPT) and extrinsic (PT) pathway of secondary hemostasis.
Acknowledgements
The authors are grateful for the financial support provided by Hakim Sabzevari University.
Authors’ Contribution
Conceptualization: Zahra Mashkani, Jafar Vatandoost.
Data curation: Zahra Mashkani.
Formal Analysis: Zahra Mashkani.
Investigation: Zahra Mashkani.
Methodology: Zahra Mashkani, Jafar Vatandoost, Toktam Hajjar, Behnam Mahdavi.
Project administration: Jafar Vatandoost.
Resources: Zahra Mashkani.
Supervision: Jafar Vatandoost.
Validation: Jafar Vatandoost, Toktam Hajjar, Behnam Mahdavi.
Visualization: Zahra Mashkani.
Writing–original draft: Zahra Mashkani.
Writing–review & editing: Jafar Vatandoost.
Competing Interests
The authors declare that there is no conflict of interests.
Ethical Approval
The present research was performed in accordance with the Guidelines for the Care and Use of Animals and was approved by the Animal Ethics Committee of Hakim Sabzevari University (IR.HSU.REC.1399.002).
Funding
This research received no grant from funding agencies in the public, commercial, or non-profit sectors.
References
- Mann KG, Orfeo T, Butenas S, Undas A, Brummel-Ziedins K. Blood coagulation dynamics in haemostasis. Hamostaseologie 2009; 29(1):7-16. [ Google Scholar]
- Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Semin Liver Dis 2002; 22(1):83-96. doi: 10.1055/s-2002-23205 [Crossref] [ Google Scholar]
- Osoniyi O, Onajobi F. Coagulant and anticoagulant activities in Jatropha curcas latex. J Ethnopharmacol 2003; 89(1):101-5. doi: 10.1016/s0378-8741(03)00263-0 [Crossref] [ Google Scholar]
- Öner AF, Doğan M, Kaya A, Sal E, Bektaş MS, Yesilmen O. New coagulant agent (ankaferd blood stopper) for open hemorrhages in hemophilia with inhibitor. Clin Appl Thromb Hemost 2010; 16(6):705-7. doi: 10.1177/1076029609337313 [Crossref] [ Google Scholar]
- Hosseinzadeh S, Jafarikukhdan A, Hosseini A, Armand R. The application of medicinal plants in traditional and modern medicine: a review of Thymus vulgaris. Int J Clin Med 2015; 6(9):635-42. doi: 10.4236/ijcm.2015.69084 [Crossref] [ Google Scholar]
- Naghdi Badi H, Makkizadeh Tafti M. Review of common thyme (Tymus vulgaris L.). J Med Plants 2003;2(7):1-12. [Persian].
- Dezfuli E, Moharrami S. Identification of chemical compounds of Thymus vulgaris essential oil. Second National Conference on Medicinal Plants and Sustainable Agriculture; 2015; Hamedan.
- Zabetian Hosseini F, Mortazavi SA, Fazli Bazaz BS, Koocheki A, Bolourian S. Study on the antimicrobial effect of Thymus vulgaris extract on the log (CFU/g) Salmonella enteritidis PT4 in mayonnaise. Iran Food Sci Technol Res J 2010;6(2):84-90. [Persian].
- Mirza M, Baher ZF. Chemical composition of essential oil from Thymus vulgaris hybrid. J Essent Oil Res 2003; 15(6):404-5. doi: 10.1080/10412905.2003.9698622 [Crossref] [ Google Scholar]
- Speck RE. Coagulation Assays and Reagents Comprising Tannin or Propyl Gallate and a Metal Ion. Google Patents; 1997.
- Bitew M, Desalegn T, Demissie TB, Belayneh A, Endale M, Eswaramoorthy R. Pharmacokinetics and drug-likeness of antidiabetic flavonoids: molecular docking and DFT study. PLoS One 2021; 16(12):e0260853. doi: 10.1371/journal.pone.0260853 [Crossref] [ Google Scholar]
- Raaof A, Al-Naqqash ZA, Jawad AL, Muhsan SM. Evaluation of the activity of crude alkaloids extracts of Zingiber officinale Roscoe, Thymus vulgaris L and Acacia arabica L as coagulant agent in lab mice. J Biomed Biotechnol 2013; 1(2):11-6. doi: 10.12691/bb-1-2-3 [Crossref] [ Google Scholar]
- Khouya T, Ramchoun M, Hmidani A, El Moualij B, Amrani S, Harnafi H. Acute toxicity and antiproliferative and procoagulant activities of fractions derived from Thymus satureioides of the Moroccan High Atlas. S Afr J Bot 2019; 121:568-76. doi: 10.1016/j.sajb.2019.01.005 [Crossref] [ Google Scholar]
- Beheshtipour J, Raeeszadeh M, Jamali R, Sistani S. The study of the effect of Medicago sativa hydroalcoholic extract on nicotine-induced liver damage in male Wistar rats. J Shahid Sadoughi Univ Med Sci 2018;25(10):759-69. [Persian].
- Jung K, Kim IH, Han D. Effect of medicinal plant extracts on forced swimming capacity in mice. J Ethnopharmacol 2004; 93(1):75-81. doi: 10.1016/j.jep.2004.03.022 [Crossref] [ Google Scholar]
- Johnson EJ, Duan JE, Srirattana K, Venkitanarayanan K, Tulman ER, Tian XC. Effects of intramuscularly injected plant-derived antimicrobials in the mouse model. Sci Rep 2022; 12(1):5937. doi: 10.1038/s41598-022-09705-9 [Crossref] [ Google Scholar]
- Gholap S, Kar A. Efficacy of some plant extracts in regulating corticosteroid-induced hyperglycaemia in mice. Pharm Biol 2003; 41(5):315-8. doi: 10.1076/phbi.41.5.315.15939 [Crossref] [ Google Scholar]
- Omidi A, Riahinia N, Montazer Torbati MB, Behdani MA. Hepatoprotective effect of Crocus sativus (saffron) petals extract against acetaminophen toxicity in male Wistar rats. Avicenna J Phytomed 2014; 4(5):330-6. [ Google Scholar]
- Shikdar S, Vashisht R, Bhattacharya PT. International normalized ratio (INR). In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2021.
- Dejana E, Callioni A, Quintana A, de Gaetano G. Bleeding time in laboratory animals II-A comparison of different assay conditions in rats. Thromb Res 1979; 15(1-2):191-7. doi: 10.1016/0049-3848(79)90064-1 [Crossref] [ Google Scholar]
- Lee RI, White PD. A clinical study of the coagulation time of blood. The American Journal of the Medical Sciences 2013; 145:495-503. [ Google Scholar]
- Brahimi M, Osmani S, Arabi A, Enta-Soltane B, Taghezout Z, Elkahili BS. The estimation of platelet count from a blood smear on the basis of the red cell: platelet ratio. Turk J Haematol 2009; 26(1):21-4. [ Google Scholar]
- Stankovic MS. Total phenolic content, flavonoid concentration and antioxidant activity of Marrubium peregrinum L extracts. Kragujevac J Sci 2011; 33:63-72. [ Google Scholar]
- Hosseinpoor Mohsen Abadi Z, Mahdavi B, Rezaei-Seresht E. Contents of aerial parts of Salvia leriifolia Benth. J Chem Health Risks 2016; 6(3):185-94. doi: 10.22034/jchr.2016.544146 [Crossref] [ Google Scholar]
- Mahdavi B, Yaacob WA, Din LB. Phytochemical study of medicinal smokes from Etlingera brevilabrum leaves. J Herb Med 2018; 13:52-62. doi: 10.1016/j.hermed.2018.04.001 [Crossref] [ Google Scholar]
- Mahdavi B, Hajar T, Ghodsi A, Mohammadhosseini M, Mehmandost M, Talebi E. Antidiabetic effect of Sophora pachycarpa seeds extract in streptozotocin-induced diabetic mice: a statistical evaluation. J Investig Med 2021; 69(6):1201-7. doi: 10.1136/jim-2021-001818 [Crossref] [ Google Scholar]
- Sobhanizadeh A, Norooziaghideh A. Evaluation of the Effect of Medicinal Plants Including Thymus vulgaris, Achillea millefolium, Mentha pulegium, Equisetum arvense, Medicago sativa, Urtica dioica and Stachys lavandulifolia on Blood Coagulation in Vitro. Deputy of research and technology: Army University of Medical Sciences; 2017.
- Bock PE, Srinivasan KR, Shore JD. Activation of intrinsic blood coagulation by ellagic acid: insoluble ellagic acid-metal ion complexes are the activating species. Biochemistry 1981; 20(25):7258-66. doi: 10.1021/bi00528a032 [Crossref] [ Google Scholar]
- Lee KW, Everts H, Kapperst HJ, Yeom KH, Beynen AC. Dietary carvacrol lowers body weight gain but improves feed conversion in female broiler chickens. J Appl Poult Res 2003; 12(4):394-9. doi: 10.1093/japr/12.4.394 [Crossref] [ Google Scholar]
- Mukherjee M, Dawson G, Sembhi K, Kakkar VV. Triglyceride dependence of factor VII coagulant activity in deep venous thrombosis. Thromb Haemost 1996; 76(4):500-1. [ Google Scholar]
- Spalletta S, Flati V, Toniato E, Di Gregorio J, Marino A, Pierdomenico L. Carvacrol reduces adipogenic differentiation by modulating autophagy and ChREBP expression. PLoS One 2018; 13(11):e0206894. doi: 10.1371/journal.pone.0206894 [Crossref] [ Google Scholar]
- Chu S, Qu W, Pang X, Sun B, Huang X. [Effect of saponin from Tribulus terrestris on hyperlipidemia]. Zhong Yao Cai 2003;26(5):341-4. [Chinese].
- Teymouri Zadeh Z, Rahimi S, Karimi Torshizi M, Omidbaigi R. A comparison between the effect of thyme, coneflower, garlic extracts and virginiamycin antibiotic on lipids serum, hematocrit percentage and hemoglobin concentration in broilers. J Med Plants 2009;8(32):37-45. [Persian].
- Yaghmaei P, Heydarian E, Poorbahman N. The effect of Thymus vulgaris aqueous extract on hyperlipidemia in streptozotocin induced diabetic Wistar rats. J Food Technol Nutr 2012;9(3):15-20. [Persian].
- Nazari A, Delghan B. Anticoagulant effect of Satureja hortensis in rats. Journal of Inflammatory Diseases 2006;9(4):14-8. [Persian].
- Hartman DA, Pontones ME, Kloss VF, Curley RW Jr, Robertson LW. Models of retinoid metabolism: microbial biotransformation of alpha-ionone and beta-ionone. J Nat Prod 1988; 51(5):947-53. doi: 10.1021/np50059a022 [Crossref] [ Google Scholar]
- Dauqan EM, Abdullah A. Medicinal and functional values of thyme (Thymus vulgaris L) herb. J Appl Biol Biotechnol 2017; 5(2):17-22. doi: 10.7324/jabb.2017.50203 [Crossref] [ Google Scholar]
- Woodward B, March BE. Effects of vitamin A on blood coagulation and clot-lysis times. Can J Physiol Pharmacol 1974; 52(5):984-90. doi: 10.1139/y74-129 [Crossref] [ Google Scholar]
- Mohagheghzadeh A, Shams‐Ardakani M, Ghannadi A. Volatile constituents of callus and flower‐bearing tops of Zataria multiflora Boiss (Lamiaceae). Flavour Fragr J 2000; 15(6):373-6. doi: 10.1002/1099-1026(200011/12)15:6<373::aidffj923>3.0.co;2-9 [Crossref] [ Google Scholar]
- El-Newary SA, Shaffie NM, Omer EA. The protection of Thymus vulgaris leaves alcoholic extract against hepatotoxicity of alcohol in rats. Asian Pac J Trop Med 2017; 10(4):361-71. doi: 10.1016/j.apjtm.2017.03.023 [Crossref] [ Google Scholar]
- Abdel Ghaffar FR, Hassouna IA, Ibrahim HM, Elelaimy IA, Abd El Latif HM. The protective effect of hesperidin or garlic oil against the hemotoxicity of diazinon in male albino rats. J Biosci Appl Res 2017; 3(1):23-36. [ Google Scholar]
- Pawlaczyk I, Czerchawski L, Kańska J, Bijak J, Capek P, Pliszczak-Król A. An acidic glycoconjugate from Lythrum salicaria L with controversial effects on haemostasis. J Ethnopharmacol 2010; 131(1):63-9. doi: 10.1016/j.jep.2010.06.001 [Crossref] [ Google Scholar]
- Yoon SJ, Pereira MS, Pavão MS, Hwang JK, Pyun YR, Mourão PA. The medicinal plant Porana volubilis contains polysaccharides with anticoagulant activity mediated by heparin cofactor II. Thromb Res 2002; 106(1):51-8. doi: 10.1016/s0049-3848(02)00071-3 [Crossref] [ Google Scholar]
- Faggio C, Morabito M, Minicante SA, Piano GL, Pagano M, Genovese G. Potential use of polysaccharides from the brown alga Undaria pinnatifida as anticoagulants. Braz Arch Biol Technol 2015; 58(5):798-804. doi: 10.1590/s1516-8913201500400 [Crossref] [ Google Scholar]
- Shim YY, An JH, Cho WD, Chun H, Kim KI, Cho HY. Inhibitory mechanism of blood coagulation and in vivo anticoagulant activities of polysaccharides isolated from Codium fragile. J Korean Soc Food Sci Nutr 2002; 31(5):917-23. [ Google Scholar]
- Cai W, Xie L, Chen Y, Zhang H. Purification, characterization and anticoagulant activity of the polysaccharides from green tea. Carbohydr Polym 2013; 92(2):1086-90. doi: 10.1016/j.carbpol.2012.10.057 [Crossref] [ Google Scholar]
- Pawlaczyk I, Czerchawski L, Pilecki W, Lamer-Zarawska E, Gancarz R. Polyphenolic-polysaccharide compounds from selected medicinal plants of Asteraceae and Rosaceaefamilies: chemical characterization and blood anticoagulant activity. Carbohydr Polym 2009; 77(3):568-75. doi: 10.1016/j.carbpol.2009.01.030 [Crossref] [ Google Scholar]
- Anoushirvani A, Moshfeghi K, Rafiee M, Bakhshi S. The comparison of efficacy of two and three-week prednisolone therapy in patients with immune thrombocytopenic purpura. J Arak Univ Med Sci 2013;15(67):9-15. [Persian].
- Atik N, Munawir MD, Tarawifa S, Darmadji HP. Effect of guava extract administration on megakaryocytes amount in mice femur. Indones J Clin Pharm 2017; 6(2):116-22. doi: 10.15416/ijcp.2017.6.2.116 [Crossref] [ Google Scholar]
- Gutti U, Komati JK, Kotipalli A, Saladi RGV, Gutti RK. Justicia adhatoda induces megakaryocyte differentiation through mitochondrial ROS generation. Phytomedicine 2018; 43:135-9. doi: 10.1016/j.phymed.2018.04.038 [Crossref] [ Google Scholar]
- Tajika K, Ikebuchi K, Inokuchi K, Hasegawa S, Dan K, Sekiguchi S. IL‐6 and SCF exert different effects on megakaryocyte maturation. Br J Haematol 1998; 100(1):105-11. doi: 10.1046/j.1365-2141.1998.00541.x [Crossref] [ Google Scholar]
- Klotoé JR, Dougnon TV, Sacramento TI, Dandjesso C, Edorh AP, Koudokpon H. Hemostatic potential of the sap of Musa sapientum L (Musaceae). J Appl Pharm Sci 2012; 2(7):65-9. doi: 10.7324/japs.2012.2707 [Crossref] [ Google Scholar]
- Tousson E, El-Moghazy M, El-Atrsh E. The possible effect of diets containing Nigella sativa and Thymus vulgaris on blood parameters and some organs structure in rabbit. Toxicol Ind Health 2011; 27(2):107-16. doi: 10.1177/0748233710381891 [Crossref] [ Google Scholar]
- Alkhamis TM, Beissinger RL, Chediak JR. Red blood cell effect on platelet adhesion and aggregation in low-stress shear flow. Myth or fact? ASAIO Trans 1988; 34(3):868-73. [ Google Scholar]
- Singh JM, Singh MD. Alkaloids of tobacco and blood coagulation: effect of nicotine on thrombin and fibrinogen. Clin Toxicol 1975; 8(1):43-52. doi: 10.3109/15563657508988045 [Crossref] [ Google Scholar]
- Forwell GD, Ingram GI. The effect of adrenaline infusion on human blood coagulation. J Physiol 1957; 135(2):371-83. doi: 10.1113/jphysiol.1957.sp005716 [Crossref] [ Google Scholar]
- Lee YY, Lee S, Jin JL, Yun-Choi HS. Platelet anti-aggregatory effects of coumarins from the roots of Angelica genuflexa and A gigas. Arch Pharm Res 2003; 26(9):723-6. doi: 10.1007/bf02976681 [Crossref] [ Google Scholar]
- Sharifi R, Hedayati M, Rasmi Y, Rahmati-Yamchi M, Fatemi F, Dadkhah A, et al. Cyclooxygenase prevention and treatment. Research in Medicine 2007;31(3):297-89. [Persian].
- Crescente M, Jessen G, Momi S, Höltje HD, Gresele P, Cerletti C. Interactions of gallic acid, resveratrol, quercetin and aspirin at the platelet cyclooxygenase-1 level Functional and modelling studies. Thromb Haemost 2009; 102(2):336-46. doi: 10.1160/th09-01-0057 [Crossref] [ Google Scholar]
- Chen CY, Milbury PE, Lapsley K, Blumberg JB. Flavonoids from almond skins are bioavailable and act synergistically with vitamins C and E to enhance hamster and human LDL resistance to oxidation. J Nutr 2005; 135(6):1366-73. doi: 10.1093/jn/135.6.1366 [Crossref] [ Google Scholar]
- Hamidpour M, Kadem Maboodi AA, Sigarchin Taghizadeh F, Janmaleki M, Seyed Alikhani SB. The influence of low-density lipoprotein on vein thrombus formation. Sci J Iran Blood Transfus Organ 2014;11(3):221-9. [Persian].
- De Caterina R, Lanza M, Manca G, Strata GB, Maffei S, Salvatore L. Bleeding time and bleeding: an analysis of the relationship of the bleeding time test with parameters of surgical bleeding. Blood 1994; 84(10):3363-70. [ Google Scholar]
- Raaof A, Al-Naqqash ZA, Jawad AL, Muhsan SM. Evaluation of the activity of crude alkaloids extracts of Zingiber officinale Roscoe, Thymus vulgaris L and Acacia arabica L as coagulant agent in lab mice. J Biomed Biotechnol 2013; 1(2):11-6. doi: 10.12691/bb-1-2-3 [Crossref] [ Google Scholar]
- Sayydi A, Ahmadi J, Asgar B, Hosseini SM. Effects of drought and slinity stresses on phenolic compounds of Thymus vulgaris. Eco-phytochemical Journal of Medical Plants 2015;2(4):50-61.[Persian].
- Mehta K, McQueen T, Tucker S, Pandita R, Aggarwal BB. Inhibition by all-trans-retinoic acid of tumor necrosis factor and nitric oxide production by peritoneal macrophages. J Leukoc Biol 1994; 55(3):336-42. doi: 10.1002/jlb.55.3.336 [Crossref] [ Google Scholar]
- Bécherel PA, Le Goff L, Ktorza S, Chosidow O, Francès C, Issaly F. CD23-mediated nitric oxide synthase pathway induction in human keratinocytes is inhibited by retinoic acid derivatives. J Invest Dermatol 1996; 106(6):1182-6. doi: 10.1111/1523-1747.ep12347939 [Crossref] [ Google Scholar]
- Iten F, Saller R, Abel G, Reichling J. Additive antimicrobial effects of the active components of the essential oil of Thymus vulgaris--chemotype carvacrol. Planta Med 2009; 75(11):1231-6. doi: 10.1055/s-0029-1185541 [Crossref] [ Google Scholar]
- Kaeidi A, Rahmani MR, Hassanshahi J. The protective effect of carvacrol and thymol as main polyphenolic compounds of thyme on some biologic systems in disease condition: a narrative review. J Rafsanjan Univ Med Sci 2020; 19(1):81-96. doi: 10.29252/jrums.19.1.81.[Persian] [Crossref] [ Google Scholar]
- Mirzaei F, Khazaei M. Role of nitric oxide in biological systems: a systematic review. J Mazandaran Univ Med Sci 2017;27(150):192-222. [Persian].
- Asgari M. Study of the Role of Nitric Oxide (NO) in Bovine Blood Coagulation in Vitro. University of Tehran; 2013. [Persian].