Avicenna Journal of Pharmaceutical Research. :57-62.
doi: 10.34172/ajpr.1075
Original Article
Combined Antibacterial Activity of Cetylpyridinium Chloride in the Presence of Two Herbal Extracts Against Streptococcus mutans
Fatemeh Hamidbeigi-Moghadam 1  , Mahshid Hoseini 1, Shirin Moradkhani 2, 3, Shabnam Pourmoslemi 1, *
, Mahshid Hoseini 1, Shirin Moradkhani 2, 3, Shabnam Pourmoslemi 1, * 
Author information:
1Department of Pharmaceutics, School of Pharmacy, Hamadan University of Medical Sciences, Hamadan, Iran
2Department of Pharmacognosy, School of Pharmacy, Hamadan University of Medical Sciences, Hamadan, Iran
3Medicinal Plants and Natural Products Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
Abstract
Background: Dental caries is one of the most prevalent chronic human infections worldwide. It begins with bacterial adherence to the tooth surface and the formation of dental plaques. Among various microorganisms involved, mutans streptococci are the principal oral microorganisms involved in the initiation and development of dental caries. Cetylpyridinium chloride (CPC), a quaternary ammonium with a rapid bactericidal effect, is widely used as the active ingredient of antiseptic oral mouthrinses. Plants extracts are also extensively employed in oral hygiene products for their antimicrobial, anti-inflammatory, and astringent properties. The present study aimed to investigate the combined antibacterial activity of CPC in combination with two plant extracts that are extensively utilized in dental hygiene products.
Methods: The aerial parts of Matricaria chamomile and hydroalcoholic extracts of Quercus infectoria galls were prepared by maceration. Dried extracts were investigated for antibacterial activity against Streptococcus mutans by determining the minimum inhibitory concentrations (MICs). A checkerboard method was applied to investigate the combined antibacterial activity of CPC in the presence of M. chamomile aerial parts and Q. infectoria gall extracts.
Results: The results of this study indicated a synergistic effect between CPC and the hydroalcoholic extract of Q. infectoria galls. However, the presence of the extract of M. chamomile aerial parts had an antagonistic effect on the antibacterial activity of CPC against S. mutans.
Conclusion: Accordingly, despite several beneficial properties, plant extracts should be cautiously used in the formulation of antimicrobial products due to the probability of unwanted antagonistic interactions that destroy the product’s efficacy.
Keywords: Antagonism, Antiseptic mouthwash, Cetylpyridinium, Herbal, Matricaria, Synergism
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Hamidbeigi-Moghadam F, Hoseini M, Moradkhani S, Pourmoslemi S. Combined antibacterial activity of cetylpyridinium chloride in the presence of two herbal extracts against Streptococcus mutans. Avicenna J Pharm Res. 2022; 3(2):57-62. doi:10.34172/ajpr.1075
Introduction
Dental caries is one of the most prevalent chronic human infections worldwide. It begins with bacterial adherence to the tooth surface and formation of dental plaque/biofilms. Cariogenic bacteria within these dental plaques produce acid by metabolizing carbohydrates. Persistent acidification drops the plaque pH under the critical pH (5.5), destroying enamel surfaces. The demineralization of the enamel and the degradation of the dentine organic matrix lead to the development of caries, and the inflammation of pulp that can end with tooth loss (1,2). Among various microorganisms, mutans streptococci, mainly Streptococcus mutans, is the principal oral microorganism involved in the initiation and development of dental caries (3). Epidemiological studies have shown a direct correlation between the salivary level of this microorganism and the number of decayed teeth. It can easily adhere to an enamel surface and form a high-cell-density biofilm by producing irreversible interactions between bacterial cells. Its other virulence factors are acidogenicity, acid tolerance, and synthesis of water-insoluble glucan from sucrose (4).
Antibacterial mouthwash formulations containing chlorhexidine, triclosan, and cetylpyridinium chloride (CPC) are usually prescribed to overcome plaque formation and subsequent complications. CPC has been studied extensively in clinical trials and is one of only two antimicrobial mouth rinse ingredients that has received a Category I recommendation from the advisory panel of the United States Food and Drug Administration for safety and effectiveness in reducing supragingival plaque and gingivitis (5). CPC is a quaternary ammonium compound with broad-spectrum antibacterial activity. It is a cationic surface-active agent with both hydrophilic and hydrophobic groups (6). This structure enables the compound to bind to the microbial cell surface and integrate into the cytoplasmic membrane. The disruption of membrane integrity results in the leakage of cytoplasmic components, eventually leading to cell death through interfering with cellular metabolism and the inhibition of cell growth (7). CPC has also been demonstrated to inhibit the plaque formation process through adsorption on the tooth enamel and interference with the co-aggregation of bacteria. This ability is important for the retention of CPC in the mouth and continued antimicrobial activity for a period of time after rinsing. Previous studies have reported significant reductions in salivary aerobic and anaerobic bacterial counts for up to seven hours following a single rinse with a CPC-containing product (8).
Apart from the above-mentioned antimicrobial agents, phytochemicals with antimicrobial and antiplaque properties are extensively used in oral hygiene products for the prevention and treatment of dental caries, as well as for antioxidant, analgesic, and elimination of bad breath effects (9,10). The incorporation of herbal products is usually associated with higher consumer acceptance due to the decreased risk of adverse effects and the promotion of antibiotic resistance (11). However, when using herbal products in combination with another antimicrobial agent, the type of interactions they have against the intended microorganisms should undergo careful investigation. The interactions observed in the case of using two or more antimicrobial agents are additive, antagonistic, or synergistic effects, predominantly based on the antibacterial mechanisms of action and the target sites of the agents. While antagonism can ruin antimicrobial therapy, the synergistic effect reduces the risk of drug resistance and side effects for the patients (12). The present study sought to evaluate the combined antibacterial activity of CPC in the presence of 2 herbal products, extracts from Matricaria chamomileaerial parts or Quercus infectoria galls, against S. mutans. Both plants are famous for their antibacterial and antiplaque ingredients and are extensively employed in commercial oral hygiene products and mouthrinses.
Materials and Methods
Materials and Strains
The standard strain of S. mutans (ATCC 35668) was obtained from the Persian Type Culture Collection of the Biotechnology Department at Iranian Research Organization for Science and Technology, Tehran, Iran. Muller-Hinton Broth (MHB) and resazurin sodium were purchased from Merck (Dartmouth, Germany) and Sigma-Aldrich (St Louis, MO, USA), respectively.
Preparation of the Extracts
The Q. infectoria galls and aerial parts of M. chamomilewere randomly collected from the wild populations of Lorestan and Hamedan Heights, respectively. The plants were dried in shade and then crushed and extracted by the hydroalcoholic solvent (80:20 v/v, ethanol/water). The extraction was performed in closed flasks on a shaker at room temperature for 24 hours. The mixtures were then filtered, and the solvent was removed by a rotary evaporator and then dried in a water bath at 50°C.
Minimum Inhibitory Concentration Determination
The minimum inhibitory concentration (MIC) of CPC and herbalextracts against S. mutans were separately determined using the micro-dilution method according to the Clinical and Laboratory Standards Institute Guideline (13). CPC was dissolved in sterile deionized water to obtain 100 μg/mL stock solution, which was further diluted to 0.1. 0.2, 0.5, 0.75, 1.5, 3.125, 6.25, 12.5, 25, and 50 μg/mL. The extracts were dissolved in sterile deionized water and serially diluted to obtain 20, 10, 5, 2.5, 1.25, 0.625, 0.312, 0.156, 0.078, and 0.039 mg/mL concentrations. The microbial inoculum was prepared by dispersing a proper amount of the colonies formed on the 24-hour culture of S. mutans in the 0.9% sodium chloride solution to obtain the turbidity equivalent to half McFarland standard. The MHB culture medium was inoculated by adding 0.1% v/v of the inoculum to obtain the final microbial concentration of 105 CFU/mL. In the 8-well columns of a microtiter plate, 100 µL of various concentrations of the extract were mixed with 100 µL of the inoculated MHB (n = 4). Inoculated and un-inoculated MHB were used as positive and negative controls, respectively. After incubating the microtiter plates at 37°C for 24 hours, the growth of the microorganism was investigated using the resazurin colorimetric test. An adequate amount of resazurin sodium salt was dissolved in distilled water to obtain a 0.01% w/v solution, which was then dispensed in the wells of the microtiter plates to the final concentration of 0.001% w/v. The plates were incubated at 37°C for 30 minutes and then assessed visually. Any color changes from purple to pink or colorless were recorded as positive microbial growth. The lowest concentration of the extract that inhibited the growth under the explained condition was considered MIC. This test was performed in triplicate, and data repeated in at least two experiments were reported as MICs.
Checkerboard Assay
The combined antimicrobial effect of CPC in the presence of the herbalextracts against S. mutans was separately determined using the checkerboard method (14). The inoculated MHB culture medium was dispensed in the wells of a microtiter plate (200 μL for a and b and 100 μL in other wells (Figure 1). The serial dilutions of CPC and the extract in sterile deionized water were poured in the wells of the microtiter plate in the order shown in Figure 1 to obtain a range of concentrations of 1/8ÍMIC to 8ÍMIC. After incubating at 37°C for 24 hours, the plates were visually investigated for microbial growth using a resazurin colorimetric assay. The well of the microtiter plate containing the lowest concentrations of both CPC and the extract that inhibited the growth of microorganism were determined. The fractional inhibitory concentration index (FICI) was calculated to evaluate the combined antimicrobial effect of CPC and the extract against S. mutans (Equation 1). FIC for CPC or the extracts was calculated by dividing its MIC in combination (A, B) by the MIC when acting alone (MICA, MICB) (15).
(1)
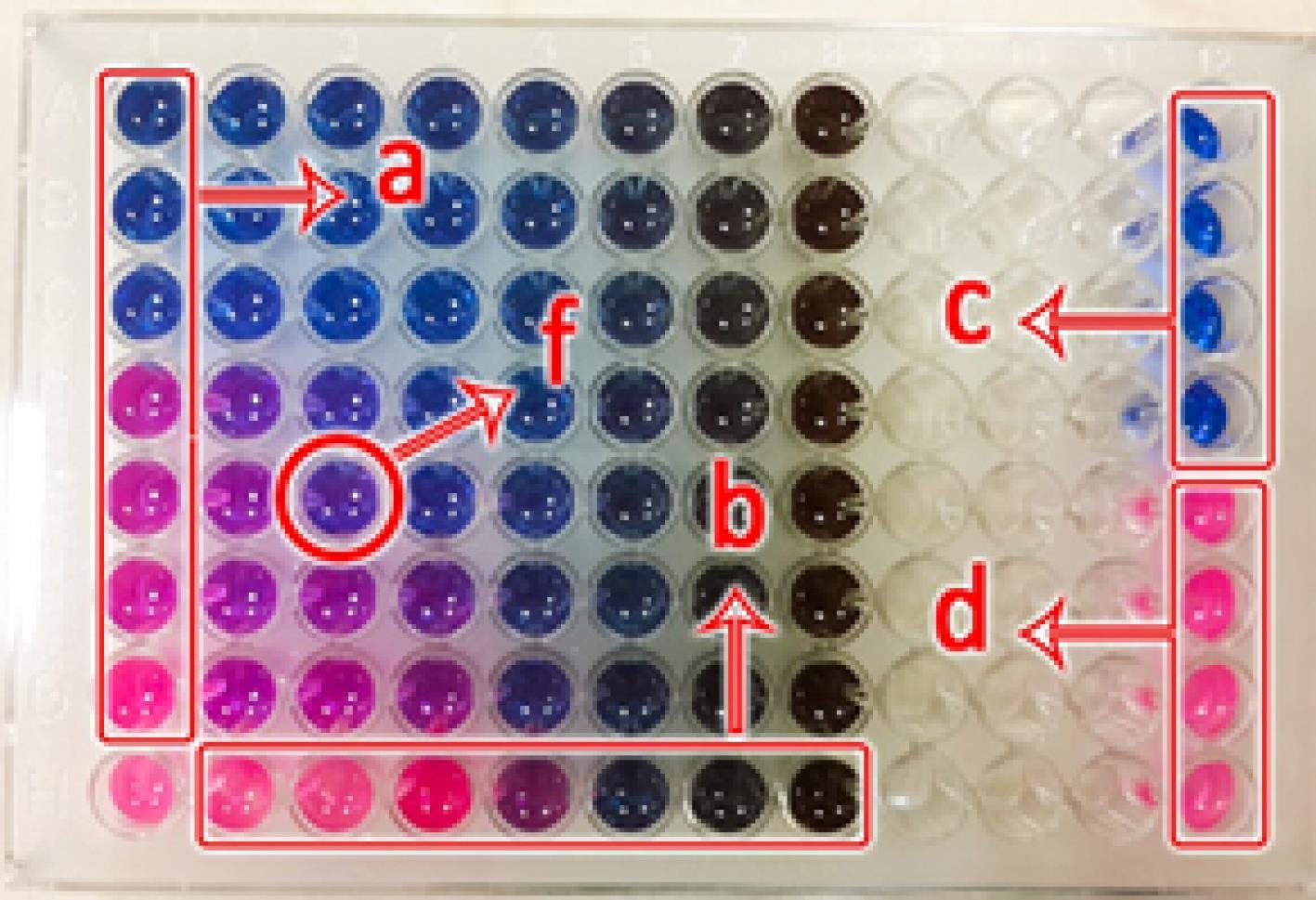
Figure 1.
Order of Adding Different Concentrations of CPC and the Extract for Checkerboard Assay: Column a: CPC, Column b: Q. infectoria Galls or Aerial Parts of M. chamomileExtracts at 1/8 to 8 fold MIC, Column c: Negative Control, Column d: Positive Control, and Well F: FIC. Note. CPC: Cetylpyridinium chloride; MIC: Minimum inhibitory concentration; Matricaria chamomile; Q. infectoria; Quercus infectoria
.
Order of Adding Different Concentrations of CPC and the Extract for Checkerboard Assay: Column a: CPC, Column b: Q. infectoria Galls or Aerial Parts of M. chamomileExtracts at 1/8 to 8 fold MIC, Column c: Negative Control, Column d: Positive Control, and Well F: FIC. Note. CPC: Cetylpyridinium chloride; MIC: Minimum inhibitory concentration; Matricaria chamomile; Q. infectoria; Quercus infectoria
Results
Determination of Minimum Inhibitory Concentration
Table 1 provides the obtained MIC amounts for CPC, M. chamomile, and Q. infectoria gall extracts. Resazurin colorimetry was used for determining the wells of microtiter plates with microbial growth. Resazurin is an oxidation-reduction indicator utilized for the determination of cell growth. Its blue non-fluorescent color changes to a fluorescent pink color after being oxidized by the enzymes of viable cells. The resazurin colorimetric method is especially useful when studying colorful and opaque substances that interfere with determining the signs of microbial growth. This method has been previously shown to be efficient and accurate for the evaluation of antibacterial herbal products (16).
Table 1.
MIC of CPC, Matricaria chamomile,and Quercus infectoria gall Extracts Against Streptococcus mutansa
|
Microorganism
|
M. chamomile
|
Q. infectoria
|
CPC
|
|
S. mutans
|
5 mg/mL |
0.312 mg/mL |
0.5 μg/mL |
Note. MIC: Minimum inhibitory concentration; CPC: Cetylpyridinium chloride.
aExperiments were performed in triplicate, and results repeated at least in two separate tests were reported as MICs.
Determination of Fractional Inhibitory Concentration Index
Table 2 summarizes MIC, FIC, and FICI amounts when CPC antibacterial activity was investigated in the presence of M. chamomile or Q. infectoria gall extracts, separately. As shown in Figure 1, in the checkerboard assay, the antibacterial activity of two agents is investigated in double serial dilutions. This technique determines the MIC of the agents alone and in combination at different concentrations. FIC and FICI values represent the ratio of changes in the MIC of each agent and both of them, respectively.
Table 2.
Combined MICs and Calculated Amounts of FIC and FICI Against Streptococcus mutansa
|
Herbal Extract
|
Extract MIC in Combination
|
FIC
|
CPC MIC in Combination
|
FIC
|
FICI
|
|
Quercus infectoria
|
0.156 |
0.5 |
0.125 |
0.25 |
0.75 |
|
Matricaria chamomile
|
5 |
1 |
1 |
2 |
3 |
Note. MIC: Minimum inhibitory concentration; FIC: Fractional inhibitory concentration; FICI: Fractional inhibitory concentration index; CPC: Cetylpyridinium chloride.
aExperiments were conducted in triplicate, and results repeated at least in two separate tests were reported as combined MICs.
Discussion
The present study was designed to investigate the antibacterial activity of Q. infectoria galls and M. chamomile herbal extracts against S. mutans as the main cariogenic microorganism. It further evaluated the type of interaction and combined antibacterial activity when using these herbal extracts in combination with CPC. Although both extracts were effective against S. mutans, the extract of Q. infectoria galls was dramatically more potent in inhibiting the growth of this microorganism. Q. infectoria is a small oak belonging to the Fagaceae family native to the Middle East and southern Europe. Spherical 1-2.5 cm in diameter galls are formed from chemical reactions after the deposition of wasps’ larvae into their young branches (17). Q. infectoria galls are mainly composed of tannins (50-70%), flavonoids, gallic acid, alkaloids, sterols, polyphenols, volatile oils, saponins, glycosides, reducing sugars, organic acids, anthraquinones, proteins, and amino acids (17-19). Some studies indicated various pharmacologic effects of Q. infectoriagalls (i.e., anti-inflammatory, anti-tumor, antioxidative and anti-radiation, cardiovascular protective, hepatoprotective, antidiabetic, and anti-bacterial activity effects). There are several reports on the anti-microbial activity of the extract of Q. infectoria galls against bacteria, yeast, and even multi-drug resistant species (20-22). Potent antibacterial activity is attributed to the presence of chemical components such as carbohydrates, lipids, mucilage, saponins, and tannins, including tannic acid with antimicrobial activity.
Matricaria chamomile L. or German chamomile is an annual plant of the composite family Asteraceae. The plant is used to treat sore stomachs and irritable bowel syndrome and is used as a gentle sleep aid. It is also employed for laxative, anti-inflammatory, and bactericidal properties, and as a mouthwash against oral mucositis (23,24). The antibacterial properties of M. chamomile are related to its phenolic, flavonoid, and flavonol compounds such as caffeic acid, eugenol, rosmarinic acid, and tannic acid (25).
Hydrolysable tannins such as tannic acid present in both Q. infectoria galls and M. chamomile extracts are considered the main antibacterial components. They effectively permeate through the cell wall peptidoglycan of Gram-positive bacteria and disrupt the cell membrane. In addition, they can inhibit the adherence of bacteria to their host cells by having structural similarity to the bacterial-binding receptors (26). More potent antibacterial activity of the extract of Q. infectoria galls is attributed to the higher content of tannins. The galls of Q. infectoria contain about 50-70% of tannins in composition that yields > 2000 mg/mL tannins concentration in the hydroalcoholic extract (The effect of extraction temperatures on tannin content). Tannins concentration is less than 200 mg/mL in the hydroalcoholic extract of M. chamomile (27).
FICI values obtained from the checkerboard assay are usually interpreted as the synergistic effect for FICI < 0.5, partial synergism for 0.5 ≤ FICI < 1, an additive effect for FICI = 1, and an antagonistic effect for FICI > 1. According to the European Committee on Antimicrobial Susceptibility Testing, when the combined activity is not greater than the sum of the activity of individual components, an additive interaction is present. Exceeding the combined activity from the sum of the individual activities is classified as synergistic interaction, and antagonistic interaction is whereby the combined activity of the components is lower than the activity of the most potent one (28). The estimated FICI values in our study demonstrated a partial synergistic interaction against S. mutans for the combination of CPC and Q. infectoria gall extract, while adding the M. chamomile extract resulted in an antagonistic effect on the CPC antibacterial activity.
Partial synergism is typically observed when the components are acting through similar mechanisms of action or on similar microbial targets (29). Membrane disruption is assumed the main antimicrobial mechanism of tannic acid as the main component of both CPC and the Q. infectoria gall extract (30). The same results have been reported by previous studies investigating the combined activity of antimicrobial agents with a similar mechanism of action. Noel et al reported additive interaction for every combination of cationic membrane-active disinfectants against a range of microorganisms. They proposed that the broadly overlapping mechanisms of antimicrobial activity provide no opportunity for a potent synergistic interaction greater than the sum of the activity of the components (29). The antagonistic effect of the M. chamomile extract on CPC antibacterial activity probably resulted from the interaction of some components of the extract with CPC that destroy its chemical structure or inhibit its antibacterial activity through chemical binding or complexation. Several previous studies have reported the same results when evaluating the combined activity of herbal products together or in the presence of pharmaceutical products. For instance, Onaku et al reported the antagonistic effect of the Carica papaya extract on the antimalarial activity of artesunic acid, the main compound in the treatment of malaria (31).
Conclusion
In recent years, the biomedical application of natural products has gained attention, and the importance of multi-target combination therapies has come to the forefront. However, the prediction of the combination effect within complex mixtures such as herbal extracts remains a challenging task. The present study aimed to examine the combined antibacterial activity of CPC in the presence of two herbal extracts that are widely used in oral hygiene products. Although both Q. infectoria galls and M. chamomile extracts showed antibacterial activities against S. mutans probably due to their tannin ingredients, totally different interactions were observed from their combinations with CPC. The partial agnostic interaction of the extract of Q. infectoria galls and CPC is the result of their same antibacterial mechanism for disrupting the bacterial cell membrane. The antagonistic effect of the M. chamomile extract may be due to the physical or chemical incompatibility of CPC with some ingredients of the extract rather than a pharmacologic interaction. It can be concluded that the complex nature of herbal products may exert different interactions when used in combination therapy products, and precise investigations are needed to prevent unwanted interactions, leading to the decreased therapeutic effect or induction of adverse effects.
Authors’ Contribution
Conceptualization: Shabnam Pourmoslemi, Shirin Moradkhani.
Data curation: Shabnam Pourmoslemi.
Formal analysis: Fatemeh Hamidbeigi-Moghadam, Mahshid Hoseini.
Funding acquisition: Fatemeh Hamidbeigi-Moghadam.
Investigation: Fatemeh Hamidbeigi-Moghadam, Mahshid Hoseini.
Methodology: Shabnam Pourmoslemi.
Project administration: Shabnam Pourmoslemi.
Resources: Shabnam Pourmoslemi.
Software: Shabnam Pourmoslemi.
Supervision: Shabnam Pourmoslemi.
Validation: Shabnam Pourmoslemi.
Visualization: Shabnam Pourmoslemi.
Writing–original draft: Fatemeh Hamidbeigi-Moghadam, Mahshid Hoseini.
Writing–review & editing: Shabnam Pourmoslemi, Shirin Moradkhani.
Competing Interests
The authors declare that they have no conflict of interests.
Ethical Approval
Not applicable.
Funding
The study was funded by Vice-chancellor for Research and Technology, Hamadan University of Medical Sciences (No. 9910307640).
References
- Wu CY, Su TY, Wang MY, Yang SF, Mar K, Hung SL. Inhibitory effects of tea catechin epigallocatechin-3-gallate against biofilms formed from Streptococcus mutans and a probiotic lactobacillus strain. Arch Oral Biol 2018; 94:69-77. doi: 10.1016/j.archoralbio.2018.06.019 [Crossref] [ Google Scholar]
- Yang Y, Liu S, He Y, Chen Z, Li M. Effect of LongZhang gargle on biofilm formation and acidogenicity of Streptococcus mutans in vitro. Biomed Res Int 2016; 2016:5829823. doi: 10.1155/2016/5829823 [Crossref] [ Google Scholar]
- Law V, Seow WK, Townsend G. Factors influencing oral colonization of mutans streptococci in young children. Aust Dent J 2007; 52(2):93-100. doi: 10.1111/j.1834-7819.2007.tb00471.x [Crossref] [ Google Scholar]
- Nishikawara F, Katsumura S, Ando A, Tamaki Y, Nakamura Y, Sato K. Correlation of cariogenic bacteria and dental caries in adults. J Oral Sci 2006; 48(4):245-51. doi: 10.2334/josnusd.48.245 [Crossref] [ Google Scholar]
- Rao D, Arvanitidou E, Du-Thumm L, Rickard AH. Efficacy of an alcohol-free CPC-containing mouthwash against oral multispecies biofilms. J Clin Dent 2011; 22(6):187-94. [ Google Scholar]
- Witt J, Ramji N, Gibb R, Dunavent J, Flood J, Barnes J. Antibacterial and antiplaque effects of a novel, alcohol-free oral rinse with cetylpyridinium chloride. J Contemp Dent Pract 2005; 6(1):1-9. [ Google Scholar]
- Busscher HJ, White DJ, Atema-Smit J, Geertsema-Doornbusch G, de Vries J, van der Mei HC. Surfactive and antibacterial activity of cetylpyridinium chloride formulations in vitro and in vivo. J Clin Periodontol 2008; 35(6):547-54. doi: 10.1111/j.1600-051X.2008.01230.x [Crossref] [ Google Scholar]
- Hu D, Li X, Sreenivasan PK, DeVizio W. A randomized, double-blind clinical study to assess the antimicrobial effects of a cetylpyridinium chloride mouth rinse on dental plaque bacteria. Clin Ther 2009; 31(11):2540-8. doi: 10.1016/j.clinthera.2009.11.004 [Crossref] [ Google Scholar]
- Dorman HJ, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol 2000; 88(2):308-16. doi: 10.1046/j.1365-2672.2000.00969.x [Crossref] [ Google Scholar]
- Dalir Abdolahinia E, Hajisadeghi S, Moayedi Banan Z, Dadgar E, Delaramifar A, Izadian S, et al. Potential applications of medicinal herbs and phytochemicals in oral and dental health: status quo and future perspectives. Oral Dis. 2022. 10.1111/odi.14276.
- Jazaeri M, Pakdek F, Rezaei-Soufi L, Abdolsamadi H, Rafieian N. Cariostatic effect of green tea in comparison with common anticariogenic agents: an in vitro study. J Dent Res Dent Clin Dent Prospects 2015; 9(1):44-8. doi: 10.15171/joddd.2015.009 [Crossref] [ Google Scholar]
- Tamri P, Pourmoslemi S, Moradkhani S, Foroughinia S. Evaluation of synergistic antibacterial effect of combined Scrophularia striata extract and antibiotics against Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus. Iraqi J Pharm Sci 2021; 30(2):219-24. doi: 10.31351/vol30iss2pp219-224 [Crossref] [ Google Scholar]
- Afhami S, Borumand MA, Esmailpour Bazzaz N, Saffar H, Hadadi A, Jafary Nezhad M. Antimicrobial resistance pattern of Acinetobacter; a multicenter study, comparing European Committee on Antimicrobial Susceptibility Testing (EUCAST) and the Clinical and Laboratory Standards Institute (CLSI); evaluation of susceptibility testing methods for polymyxin. Immunopathol Persa 2021; 7(1):e04. doi: 10.34172/ipp.2021.04 [Crossref] [ Google Scholar]
- Kemegne GA, Sado Kamdem SL, Nyegue MA, Menut C, Etoa FX. Comparing checkerboard, isobologram and CCD methods for drug combination: a case study of ciprofloxacin and plant extracts on Escherichia coli and Shigella. J Med Plants Res 2021; 5(10):479-89. doi: 10.5897/jmpr2021.7139 [Crossref] [ Google Scholar]
- Apridamayanti P, Sari R, Rachmaningtyas A, Aranthi V. Antioxidant, antibacterial activity and FICI (Fractional Inhibitory Concentration Index) of ethanolic extract of Melastomamalabathricum leaves with amoxicillin against pathogenic bacteria. Nusantara Biosci 2021; 13(2):140-7. doi: 10.13057/nusbiosci/n130202 [Crossref] [ Google Scholar]
- Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007; 42(4):321-4. doi: 10.1016/j.ymeth.2007.01.006 [Crossref] [ Google Scholar]
- Elham A, Arken M, Kalimanjan G, Arkin A, Iminjan M. A review of the phytochemical, pharmacological, pharmacokinetic, and toxicological evaluation of Quercus infectoria galls. Journal of Ethnopharmacology 2021; 273:113592. doi: 10.1016/j.jep.2020.113592 [Crossref] [ Google Scholar]
- Onal A, Sari A, Soylak M. Ellagic acid from gallnut (Quercus infectoria): extraction and determination of its dyeing conditions for natural fibres. J Sci Ind Res 2005; 64(7):491-5. [ Google Scholar]
- Chusri S, Voravuthikunchai SP. Detailed studies on Quercus infectoria Olivier (nutgalls) as an alternative treatment for methicillin-resistant Staphylococcus aureus infections. J Appl Microbiol 2009; 106(1):89-96. doi: 10.1111/j.1365-2672.2008.03979.x [Crossref] [ Google Scholar]
- Basri DF, Tan LS, Shafiei Z, Zin NM. In vitro antibacterial activity of galls of Quercus infectoria Olivier against oral pathogens. Evid Based Complement Alternat Med 2012; 2012:632796. doi: 10.1155/2012/632796 [Crossref] [ Google Scholar]
- Baharuddin NS, Abdullah H, Abdul Wahab WN. Anti-Candida activity of Quercus infectoria gall extracts against Candida species. J Pharm Bioallied Sci 2015; 7(1):15-20. doi: 10.4103/0975-7406.148742 [Crossref] [ Google Scholar]
- Shao D, Li J, Li J, Tang R, Liu L, Shi J. Inhibition of gallic acid on the growth and biofilm formation of Escherichia coli and Streptococcus mutans. J Food Sci 2015; 80(6):M1299-305. doi: 10.1111/1750-3841.12902 [Crossref] [ Google Scholar]
- Munir N, Iqbal AS, Altaf I, Bashir R, Sharif N, Saleem F. Evaluation of antioxidant and antimicrobial potential of two endangered plant species Atropa belladonna and Matricaria chamomilla. Afr J Tradit Complement Altern Med 2014; 11(5):111-7. doi: 10.4314/ajtcam.v11i5.18 [Crossref] [ Google Scholar]
- Motavalizadehkakhky A. Antimicrobial activity and chemical composition of essential oils of chamomile from Neyshabur, Iran. J Med Plants Res 2012; 6(5):820-4. [ Google Scholar]
- Abdoul-Latif FM, Mohamed N, Edou P, Ali AA, Djama SO, Obame LC. Antimicrobial and antioxidant activities of essential oil and methanol extract of Matricaria chamomilla L from Djibouti. J Med Plants Res 2011; 5(9):1512-7. [ Google Scholar]
- Mustafa H, Ismail N, Wahab W. Anti-microbial activity of aqueous Quercus infectoria gall extract against pathogenic Leptospira. Malays J Med Sci 2018; 25(4):42-50. doi: 10.21315/mjms2018.25.4.4 [Crossref] [ Google Scholar]
- Seddik K, Dalila B, Saliha D, Saliha D, Smain A, Noureddine C. Polyphenols and antioxidant properties of extracts from Mentha pulegium L and Matricaria chamomilla L. Pharmacogn Commun 2013; 3(2):35-40. doi: 10.5530/pc.2013.2.8 [Crossref] [ Google Scholar]
- EUCAST EUCAST. European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID): terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin Microbiol Infect 2003; 9:1-7. [ Google Scholar]
- Noel DJ, Keevil CW, Wilks SA. Synergism versus additivity: defining the interactions between common disinfectants. mBio 2021; 12(5):e0228121. doi: 10.1128/mBio.02281-21 [Crossref] [ Google Scholar]
- Mao X, Auer DL, Buchalla W, Hiller KA, Maisch T, Hellwig E, et al. Cetylpyridinium chloride: mechanism of action, antimicrobial efficacy in biofilms, and potential risks of resistance. Antimicrob Agents Chemother 2020;64(8). 10.1128/aac.00576-20.
- Onaku LO, Attama AA, Okore VC, Tijani AY, Ngene AA, Esimone CO. Antagonistic antimalarial properties of pawpaw leaf aqueous extract in combination with artesunic acid in Plasmodium berghei-infected mice. J Vector Borne Dis 2011; 48(2):96-100. [ Google Scholar]