Avicenna Journal of Pharmaceutical Research. :82-90.
doi: 10.34172/ajpr.1068
Original Article
Novel 2-Amino-pyrano[3,2-c]quinoline-3-carbonitrile Derivatives Bearing Benzyloxy Phenyl Moiety as Butyrylcholinesterase Inhibitors: Design, Synthesis, In Vitro Evaluation, and Molecular Docking Studies
Gholamabbas Chehardoli 1, Fatemeh Karimi 2, Tahmineh Akbarzadeh 3, 4, Roshanak Hariri 3, 4, Zahra Najafi 2, * 
Author information:
1Department of Medicinal Chemistry, School of Pharmacy, Medicinal Plants and Natural Products Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
2Department of Medicinal Chemistry, School of Pharmacy, Hamadan University of Medical Sciences, Hamadan, Iran
3Department of Medicinal Chemistry, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
4Persian Medicine and Pharmacy Research Center, Tehran University of Medical Sciences, Tehran, Iran
Abstract
Background: Alzheimer’s disease (AD), the main form of dementia, is a multifactorial neurodegenerative disease, and several hypotheses have been proposed for its pathogenesis. Among them, cholinergic hypofunction is the main reason and plays a significant role in cognitive impairment. According to this theory, ChE inhibitors improve the performance of the cholinergic system and increase memory function. Thus, this study investigated a novel series of 2-amino-pyrano[3,2-c]quinoline-3-carbonitrile derivatives bearing benzyloxy phenyl moiety as ChE enzyme inhibitors.
Methods: The synthesized compounds 6a-o are divided into three series based on benzyloxy phenyl moiety. The structure of all compounds was identified by the NMR (1H and 13C) and IR spectra. Then, their inhibitory activities against ChE enzymes were evaluated by Ellman’s spectrophotometrical method. The kinetic and molecular docking studies were performed for compound 6l as the most potent butyrylcholinesterase (BChE) inhibitor.
Results: The 2-amino-4-(4-((4-fluorobenzyl)oxy)-3-methoxyphenyl)-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (6l) demonstrated the best anti-BChE activity with a half maximal inhibitory concentration value of 1.00±0.07. The kinetic and molecular docking studies confirmed that 6l is a mixed inhibitor and binds to both the anionic catalytic site and peripheral anionic site (PAS) of BChE. In silico study approved that the methoxy group on the middle phenyl ring has a significant role in interacting with the PAS of the enzyme.
Conclusion: These findings indicated that 2-amino-pyrano[3,2-c]quinoline-3-carbonitrile derivatives bearing benzyloxy phenyl moiety have therapeutic potential as BChE inhibitors in the last stages of AD.
Keywords: Pyran, Quinoline, Synthesis, Molecular docking, Cholinesterase inhibitors, Alzheimer’s disease
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Chehardoli G, Karimi F, Akbarzadeh T, Hariri R, Najafi Z. Novel 2-Amino-pyrano[3,2-c]quinoline-3-carbonitrile derivatives bearing benzyloxy phenyl moiety as butyrylcholinesterase inhibitors: design, synthesis, In vitro evaluation, and molecular docking studies. Avicenna J Pharm Res. 2022; 3(2):82-90. doi:10.34172/ajpr.1068
Introduction
Alzheimer’s disease (AD) is one of the most common forms of dementia in adults that appears with a decrease in cognitive functions. Alzheimer’s is a multifactorial disease, and scientists have proposed several reasons and hypotheses for the pathogenesis of the disease, including environmental factors such as head trauma, aluminum exposure and malnutrition, genetic agent (1), and several hypotheses, including amyloid aggregation (2), cholinergic hypofunction (3), Cortico-cortical pathways (4), mitochondrial dysfunction (5), and neuroinflammation (6). Among them, cholinergic signaling plays a significant role in cognitive function and is widely distributed in the basal forebrain (7). Acetylcholine (ACh) is a neurotransmitter and modulator in the cholinergic neurotransmission system, which is responsible for regulating important biological processes such as memory, learning, stress response, sensory information, sleep, and wakefulness (8). ACh is hydrolytically degraded in the brain by two cholinesterases, namely, acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). To date, cholinesterase inhibitors (ChEIs) such as tacrine, donepezil, rivastigmine, and galantamine (Figure 1) have been approved for the treatment of AD (9,10). In patients with AD, a misbalance takes place between AChE and BChE so that the activity of AChE does not change or decrease, whereas BChE activity increases significantly. Therefore, it seems that the inhibition of BChE could also be considered a valuable therapeutic target for the treatment of AD (11). In recent years, medicinal chemists have represented great interest in designing and synthesizing a new hybrid molecule of two or more different pharmacophores that have different biological properties and structures (12). Thus, the hybridization method has grown to be a golden approach to drug discovery. Pyran and quinoline rings are valuable structures in medicinal chemistry with a wide range of biological functions such as anticancer, antimalarial, antibacterial, antifungal, antituberculosis, and anti-Alzheimer properties (13-15). These pharmaceutical and biological activities of pyran and quinoline rings, along with the presence of benzyl moiety in ChEIs (Figure 2) (16-21), encouraged us to design novel hybrid molecules. As a result, in continuation of our research programs on the synthesis of ChEIs (16,22), this study aimed to synthesize and evaluate novel 2-amino-pyrano[3,2-c]quinoline-3-carbonitrile derivatives bearing benzyloxy phenyl moiety as BChEIs.
Figure 1.
Structures of (a) Tacrine, (b) Rivastigmine, (c) Donepezil, and (d) Galantamine as AChEIs. Note. AChEI: Acetylcholinesterase inhibitor
.
Structures of (a) Tacrine, (b) Rivastigmine, (c) Donepezil, and (d) Galantamine as AChEIs. Note. AChEI: Acetylcholinesterase inhibitor
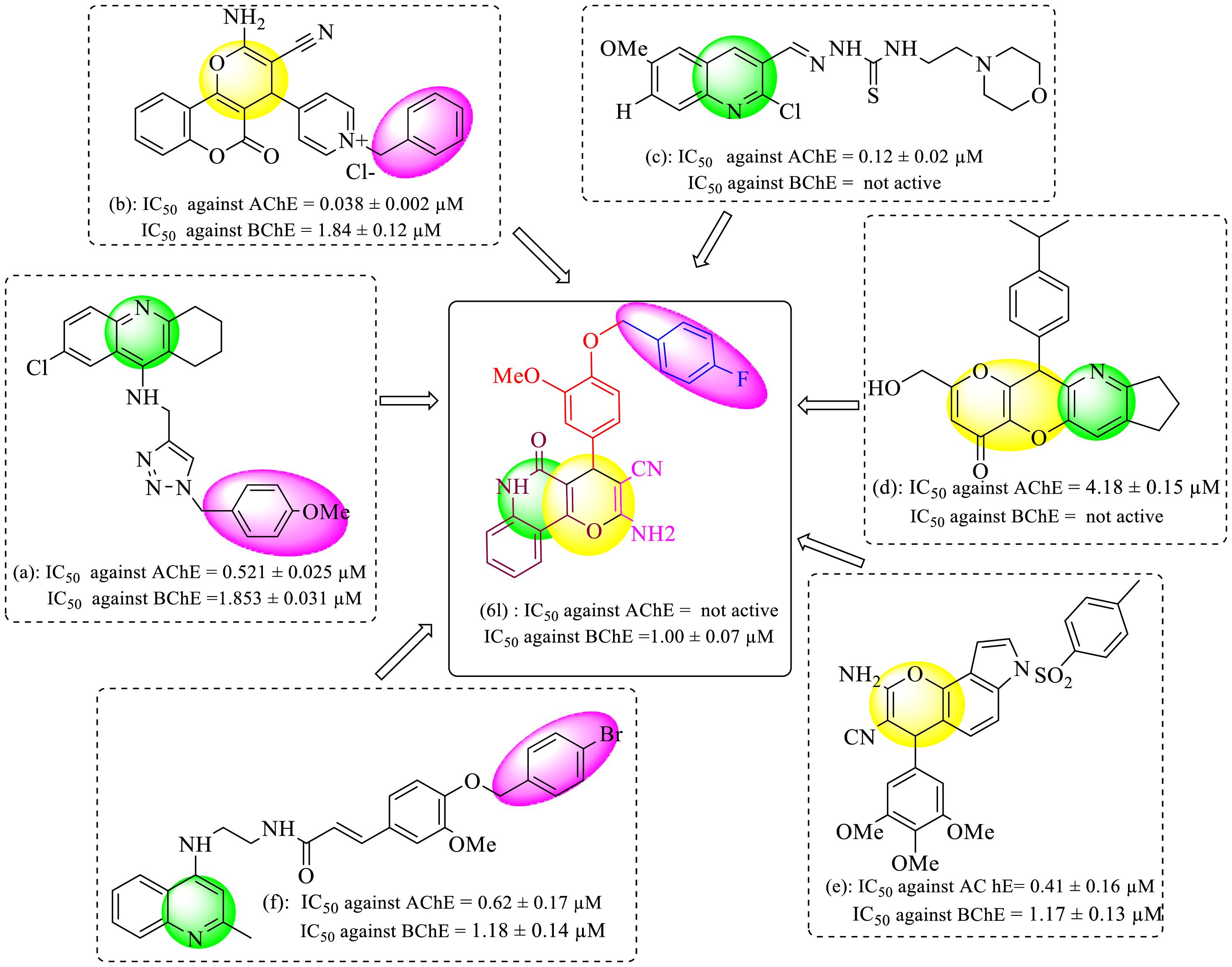
Figure 2.
The Structures of Some Pyran and Quinoline-based Hybrid molecules as AChE and BChE Inhibitors and Designed Compound 6l. Note. AChE: Acetylcholinesterase; BChE: Butyrylcholinesterase
.
The Structures of Some Pyran and Quinoline-based Hybrid molecules as AChE and BChE Inhibitors and Designed Compound 6l. Note. AChE: Acetylcholinesterase; BChE: Butyrylcholinesterase
Methods
All chemicals were obtained from Merck and Sigma Companies and were used without further purification. Melting points were measured on a Stuart melting point smp3 apparatus. The nuclear magnetic resonance (NMR; 1H and 13C) and infrared (IR) spectra were obtained by using a Bruker 400-NMR and ALPHA Fourier-transform infrared spectrometer on KBr disks, respectively. The chemical shifts (δ) and coupling constants (J) are expressed in parts per million (ppm) and Hertz, respectively. The atom numbering of the target compounds was performed based on the IUPAC name and used to assign the 1H-NMR data. The original spectra of the investigated compounds are provided as Suplementary file.
Chemistry
General Procedure for the Synthesis of Benzyloxy Aldehydes Derivatives (3a-o):
4-Hydroxy benzaldehyde, 3-hydroxy benzaldehyde, and 3-methoxy-4-hydroxy benzaldehyde (vanillin) (1 mmol) were reacted with various benzyl halide (1.2 mmol) derivatives in the presence of K2CO3 (1.5 mmol) and DMF (5 mL). After the reaction was completed, cold ice-water was added to the mixture of the reaction. Benzyloxy aldehydes were obtained as white precipitates and used for the next stage without further purification (23).
General Procedure for the Synthesis of New Benzyloxy Pyrano[3,2-c]quinoline-3-carbonitrile Derivatives (6a-o)
The benzyloxy aldehydes (1 mmol) of the previous step, malononitrile 0.066 g (1 mmol), and 4-hydroxyquinolin-2(1H)-one 0.16g (1 mmol) were added to the round-bottomed flask in the presence of a catalytic amount of NH4OAc in ethanol (80°C) as a solvent (5 mL) and refluxed for 12 hours. The progress of the reaction was followed by the thin layer chromatography technique. After the completion of the reaction, the mixture of reaction cold to room temperature and resulting precipitates were simply filtered and washed with 70% ethanol (24).
2-Amino-4-(4-(benzyloxy)phenyl)-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (6a)
White solid; Mp: 236-237 °C; IR (KBr): υ (cm-1) = 3386, 3327,3207, 2874, 2199, 1677. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.76 (s, 1H, NH), 7.99 (d, J = 8.8 Hz, 1H, H10), 7.58 (t, J = 8.8 Hz, 1H, H9), 7.48 (d, J = 8.8 Hz, 1H, H7), 7.45 – 7.35 (m, 6H, H2’’, H3’’, H4’’, H5’’, H6’’ &H8), 7.24 (s, 2H, NH2), 7.13 (d, J = 8.7 Hz, 2H, H2’& H6’), 6.93 (d, J = 8.7 Hz, 2H, H3’& H5’), 5.06 (s, 2H, CH2), 4.45 (s, 1H, H4). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 163.4, 160.4, 158.9, 157.2, 150.9, 137.7, 137.1, 136.7, 133.3, 131.1, 128.4, 128.0, 127.6, 121.9, 119.9, 115.9, 115.3, 114.5, 112.0, 109.8, 69.2, 57.9, 35.9.Anal. calcd. for C26H19N3O3: C, 74.1; H, 4.54; N, 9.97. Found: C, 73.93; H, 4.68; N, 9.80.
2-Amino-4-(4-((4-fluorobenzyl)oxy)phenyl)-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (6b)
White solid; Mp: 253-254 °C; IR (KBr): υ (cm-1) = 3460, 3327, 3210, 2910, 2203, 1683. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.78 (s, 1H, NH), 7.91 (d, J = 8.0 Hz, 1H, H10), 7.58 (t, J = 8.0 Hz, 1H, H9), 7.48 (d, J = 8.6, Hz, 2H, H3’’& H5’’ ), 7.34 (d, J = 8.0 Hz, 1H, H7), 7.30 (t, J = 8.0 Hz, 1H, H8), 7.25 (s, 2H, NH2), 7.20 (d, J = 8.6 Hz, 2H, H2’’& H6’’), 7.14 (d, J = 8.6 Hz, 2H, H2’& H6’), 6.93 (d, J = 8.6 Hz, 2H, H3’& H5’), 5.04 (s, 2H, CH2), 4.46 (s, 1H, H4). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 162.9, 160.5, 160.4, 158.9, 157.1, 150.9, 137.7, 136.7, 133.3, 133.3, 131.1, 129.9, 129.8, 128.5, 121.9, 121.7, 119.9, 115.3, 115.3, 115.1, 114.5, 112.0, 109.8, 68.4, 57.9, 35.9.Anal. calcd. for C26H18FN3O3: C, 71.06; H, 4.13; N, 9.56. Found: C, 71.23; H, 4.01; N, 9.62.
2-Amino-4-(4-((4-chlorobenzyl)oxy)phenyl)-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (6c)
White solid; Mp: 270-271 °C; IR (KBr): υ (cm-1) = 3456, 3327, 3203, 2888, 2203, 1691. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.77 (s, 1H, NH), 7.90 (d, J = 7.7 Hz, 1H, H10), 7.58 (t, J = 7.7 Hz, 1H, H9), 7.46 (s, 4H, H2’’, H3’’, H5’’, H6’’ ), 7.36 – 7.29 (m, 2H, H7&H8), 7.25 (s, 2H, NH2), 7.13 (d, J = 8.7 Hz, 2H, H3’& H5’), 6.92 (d, J = 8.7 Hz, 2H, H2’& H6’), 5.06 (s, 2H, H4), 4.45 (s, 1H, CH2). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 160.4, 158.8, 157.0, 150.9, 137.7, 136.8, 136.2, 132.3, 131.1, 129.4, 128.5, 128.4, 121.9, 121.7, 119.9, 115.3, 114.5, 112.0, 109.8, 68.3, 57.9, 35.9.Anal. calcd. for C26H18ClN3O3: C, 68.50; H, 3.98; N, 9.22. Found: C, 68.42; H, 4.11; N, 9.28.
2-Amino-4-(4-((4-bromobenzyl)oxy)phenyl)-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (6d)
White solid; Mp: 241-242 °C; IR (KBr): υ (cm-1) = 3456, 3323, 3205, 2877, 2201, 1683. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.77 (s, 1H, NH), 7.99 (d, J = 9.0 Hz, 2H, H3’’, H5’’), 7.90 (d, J = 8.1 Hz, 1H, H10), 7.67 – 7.56 (m, 3H, H9, H2’’, H6’’ ), 7.40 (d, J = 8.1 Hz, 1H, H7), 7.32 (t, J = 8.1 Hz, 1H,H8), 7.25 (s, 2H, NH2), 7.13 (d, J = 8.7 Hz, 2H, H3’, H5’), 6.92 (d, J = 8.7 Hz, 2H, H2’, H6’), 5.04 (s, 2H, CH2), 4.45 (s, 1H, H4). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 163.1, 160.4, 160.4, 158.8, 157.0, 150.9, 137.7, 135.5, 133.3, 131.5, 130.1, 129.7, 128.5, 124.4, 115.9, 114.5, 109.8, 69.0, 57.9, 35.9. Anal. calcd. for C26H18BrN3O3: C, 62.41; H, 3.63; N, 9.34. Found: C, 62.52; H, 3.51; N, 9.28.
2-Amino-4-(4-((4-methylbenzyl)oxy)phenyl)-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (6e)
White solid; Mp: 254-255 °C; IR (KBr): υ (cm-1) = 3441, 3325, 3199, 2205, 1672. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.77 (s, 1H, NH), 7.91 (d, J = 8.1 Hz, 1H, H10), 7.58 (t, J = 8.1 Hz, 1H, H9), 7.34 (d, J = 8.1 Hz, 1H, H7), 7.32 – 7.28 (m, 3H, H8, H3’’& H5’’ ), 7.25 (s, 2H, NH2), 7.18 (d, J = 7.8 Hz, 2H, H2’’& H6’’), 7.12 (d, J = 8.7 Hz, 2H, H2’& H6’), 6.91 (d, J = 8.7 Hz, 2H, H3’& H5’), 5.00 (s, 2H, CH2), 4.45 (s, 1H, H4), 2.30 (s, 3H, CH3). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 160.4, 158.9, 157.2, 150.9, 137.7, 137.0, 136.6, 134.0, 131.1, 128.9, 128.4, 127.7, 121.9, 121.7, 119.9, 115.3, 114.5, 112.0, 109.8, 69.0, 57.9, 35.9, 20.7. Anal. calcd. for C27H21N3O3: C, 72.47; H, 4.86; N, 9.65. Found: C, 72.61; H, 4.72; N, 9.78.
2-Amino-4-(3-(benzyloxy)phenyl)-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (6f)
White solid; Mp: 255-256 °C; IR (KBr): υ (cm-1) = 3464, 3335, 3209, 3041, 2203, 1683. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.79 (s, 1H, NH), 7.92 (d, J = 8.1 Hz, 1H, H10), 7.60 (t, J = 8.1 Hz, 1H, H9), 7.42 (d, J = 6.6 Hz, 2H, H3’’, H5’’), 7.35 (d, J = 6.6,Hz, 2H, H2’’, H6’’), 7.33 – 7.31 (m, 2H, H7&H8), 7.29 (s, 2H, NH2), 7.22 (t, J = 8.1 Hz, 1H, H5’), 6.90 – 6.86 (m, 1H, H6’), 6.82 – 6.78 (m, 2H, H2’& H4’), 5.03 (s, 2H, CH2), 4.48 (s, 1H, H4). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 160.4, 159.0, 158.3, 151.2, 145.9, 137.7, 136.9, 131.2, 129.5, 128.3, 127.8, 127.8, 122.0, 121.7, 119.8, 115.3, 114.2, 112.4, 112.0, 109.4, 69.2, 57.5, 36.5.Anal. calcd. for C26H19N3O3: C, 74.1; H, 4.54; N, 9.97. Found: C, 74.23; H, 4.68; N, 9.75.
2-Amino-4-(3-((4-fluorobenzyl)oxy)phenyl)-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (6g)
White solid; Mp: 267-268 °C; IR (KBr): υ (cm-1) = 3505,3398, 3203, 2907, 2193, 1683. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.80 (s, 1H,NH), 7.92 (d, J = 8.1 Hz, 1H, H10), 7.60 (t, J = 8.1 Hz, 1H, H9), 7.48 (d, J = 8.6, Hz, 2H, H2’’, H6’’), 7.35 (d, J = 8.1 Hz, 1H, H7), 7.33 – 7.30 (m, 1H, H5’), 7.29 (s, 2H, NH2), 7.22 (t, J = 8.1 Hz, 1H, H8), 7.16 (t, J = 8.6 Hz, 2H, H3’’, H5’’), 6.90 – 6.86 (m, 1H, H6’), 6.82 – 6.77 (m, 2H,, H2’& H4’ ), 5.02 (s, 2H, CH2), 4.48 (s, 1H, H4). 13C NMR (101 MHz, DMSO-d6) δ (ppm):13C NMR (101 MHz, DMSO) δ 162.9, 160.5, 160.4, 159.0, 158.2, 151.2, 145.9, 137.7, 133.1, 133.1, 131.2, 130.1, 130.0, 129.5, 122.0, 121.7, 119.9, 119.8, 115.3, 115.2, 115.0, 114.2, 112.4, 111.9, 109.4, 68.4, 57.5, 36.5. Anal. calcd. for C26H18FN3O3: C, 71.06; H, 4.13; N, 9.56. Found: C, 71.13; H, 4.19; N, 9.42.
2-Amino-4-(3-((4-chlorobenzyl)oxy)phenyl)-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (6h)
White solid; Mp: 258-259 °C; IR (KBr): υ (cm-1) = 3498, 3380, 3274, 2952, 2193, 1683. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.81 (s, 1H, NH), 7.93 (d, J = 8.0 Hz, 1H, H10), 7.60 (t, J = 8.0 Hz, 1H, H9), 7.46 (d, J = 8.0 Hz, 1H, H7), 7.41-7.45 (m, 2H), 7.37-7.39 (m, 1H, H8), 7.37 – 7.27 (m, 4H, H2’’, H3’’, H5’’, H6’’), 7.23 (t, J = 8.1 Hz, 1H, H5’), 6.88 (dd, J = 8.2, 2.2 Hz, 1H, H6’), 6.83 (d, J = 3.4 Hz, 1H, H4’), 6.82 (d, J = 2.2 Hz, 1H, H2’), 5.05 (s, 2H, CH2), 4.50 (s, 1H, H4). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 160.9, 159.5, 158.7, 151.7, 146.5, 138.3, 136.5, 132.8, 131.7, 130.1, 130.0, 128.8, 122.5, 122.3, 120.5, 120.3, 115.9, 114.7, 112.9, 112.5, 109.9, 68.8, 58.0, 37.1. Anal. calcd. for C26H18ClN3O3: C, 68.50; H, 3.98; N, 9.22. Found: C, 68.62; H, 4.16; N, 9.08.
2-Amino-4-(3-((4-bromobenzyl)oxy)phenyl)-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (6i)
White solid; Mp: 259-260 °C; IR (KBr): υ (cm-1) = 3500, 3370, 3184, 2935, 2193, 1675. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.79 (s, 1H, NH), 7.92 (d, J = 8.0 Hz, 1H, H10), 7.60 (t, J = 8.0 Hz, 1H, H9), 7.53 (d, J = 8.4 Hz, 2H, H3’’, H5’’), 7.39 (d, J = 8.4 Hz, 2H, H2’’, H6’’), 7.35 (d, J = 8.0 Hz, 1H, H7), 7.32 (t, J = 8.0 Hz, 1H, H8), 7.29 (s, 2H, NH2), 7.22 (t, J = 8.1 Hz, 1H, H5’), 6.88 – 6.85 (m, 1H, H6’), 6.82 – 6.79 (m, 2H, H2’& H4’), 5.02 (s, 2H, CH2), 4.49 (s, 1H, H4). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 160.4, 159.0, 158.1, 151.2, 146.0, 137.7, 136.4, 131.2, 131.2, 129.9, 129.5, 122.0, 121.7, 120.9, 119.9, 119.8, 115.3, 114.2, 112.4, 112.0, 109.4, 68.3, 57.5, 36.5. Anal. calcd. for C26H18BrN3O3: C, 62.41; H, 3.63; N, 9.34. Found: C, 62.32; H, 3.71; N, 9.48.
2-Amino-4-(3-((4-methylbenzyl)oxy)phenyl)-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (6j)
White solid; Mp: 263-264 °C; IR (KBr): υ (cm-1) = 3470, 3345, 2890, 2197, 1683. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.79 (s, 1H, NH), 7.92 (d, J = 8.0 Hz, 1H,, H10), 7.60 (t, J = 8.0 Hz, 1H, H9), 7.35 (d, J = 8.0 Hz, 1H, H7), 7.32 – 7.30 (m, 1H, H8), 7.30 – 7.28 (m, 4H, H2’’, H6’’ &NH2), 7.21 (t, J = 8.2 Hz, 1H, H5’), 7.13 (d, J = 7.7 Hz, 2H, H3’’, H5’’), 6.88 – 6.84 (m, 1H, H6’), 6.80 – 6.76 (m, 2H, H2’& H4’), 4.98 (s, 2H, CH2), 4.47 (s, 1H, H4), 2.28 (s, 3H, CH3). 13C NMR (101 MHz, DMSO-d6) δ (ppm):13C NMR (101 MHz, DMSO) δ 160.4, 159.0, 158.4, 151.2, 145.9, 137.7, 137.0, 133.8, 131.2, 129.4, 128.9, 127.9, 122.0, 121.7, 119.8, 119.7, 115.3, 114.1, 112.5, 112.0, 109.4, 69.0, 57.5, 36.5, 20.7. Anal. calcd. for C27H21N3O3: C, 72.47; H, 4.86; N, 9.65. Found: C, 72.41; H, 4.65; N, 9.49.
2-Amino-4-(4-(benzyloxy)-3-methoxyphenyl)-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (6k)
White solid; Mp: 244-245 °C; IR (KBr): υ (cm-1) 3464, 3337, 3199, 2872, 2201, 1685, 1379. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.79 (s, 1H, NH), 7.91 (d, J = 8.1 Hz, 1H, H10), 7.58 (t, J = 8.1 Hz, 1H, H9), 7.45 – 7.30 (m, 7H, H2’’, H3’’, H4’’, H5’’, H6’’, H7, H8 ), 7.25 (s, 2H, NH2), 6.95 (d, J = 8.4 Hz, 1H, H5’), 6.89 (d, J = 2.1 Hz, 1H, H2’), 6.66 (dd, J = 8.4, 2.1 Hz, 1H, H6’), 5.03 (s, 2H, CH2), 4.47 (s, 1H, H4), 3.73 (s, 3H, OMe). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 160.5, 159.0, 151.0, 148.7, 146.7, 137.7, 137.3, 137.2, 131.1, 128.4, 127.8, 127.7, 121.9, 121.7, 119.9, 119.1, 115.3, 113.5, 112.0, 111.8, 109.7, 69.9, 57.7, 55.5, 36.1. Anal. calcd. for C26H19N3O3: C, 74.1; H, 4.54; N, 9.97. Found: C, 73.98; H, 4.48; N, 9.83.
2-Amino-4-(4-((4-fluorobenzyl)oxy)-3-methoxyphenyl)-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (6l)
White solid; Mp: 243-244 °C; IR (KBr): υ (cm-1) = 3476, 3352, 2895, 2201, 1685. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.78 (s, 1H, NH), 7.91 (d, J = 8.1 Hz, 1H, H10), 7.58 (t, J = 8.1 Hz, 1H, H9), 7.48 (d, J = 8.6, Hz, 2H, H2’’, H6’’), 7.34 (d, J = 8.1 Hz, 1H, H7), 7.58 (t, J = 8.1 Hz, 1H, H8), 7.25 (s, 2H, NH2), 7.21 (t, J = 8.6 Hz, 2H, H3’’, H5’’), 6.95 (d, J = 8.3 Hz, 1H, H5’), 6.89 (d, J = 2.1 Hz, 1H, H2’), 6.66 (dd, J = 8.3, 2.1 Hz, 1H, H6’), 5.01 (s, 2H, CH2), 4.47 (s, 1H, H4), 3.73 (s, 3H, OMe). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 162.9, 160.6, 160.5, 159.0, 151.0, 148.7, 146.6, 137.7, 137.4, 133.4, 133.4, 131.1, 130.4, 129.9, 129.9, 121.9, 121.7, 119.9, 119.1, 115.3, 115.1, 113.6, 112.0, 111.8, 109.7, 69.2, 57.7, 55.5, 36.1. Anal. calcd. for C26H18FN3O3: C, 71.06; H, 4.13; N, 9.56. Found: C, 71.20; H, 4.16; N, 9.40.
2-Amino-4-(4-((4-chlorobenzyl)oxy)-3-methoxyphenyl)-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (6m)
White solid; Mp: 258-259 °C; IR (KBr): υ (cm-1) = 3433, 3343, 3207, 2909, 2197, 1687. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.78 (s, 1H, NH), 7.90 (d, J = 8.1 Hz, 1H, H10), 7.58 (t, J = 8.1 Hz, 1H, H9), 7.45 (s, 4H, H2’’, H3’’, H5’’, H6’’), 7.34 (d, J = 8.1 Hz, 1H, H7), 7.30 (t, J = 8.1 Hz, 1H, H8), 7.25 (s, 2H, NH2), 6.93 (d, J = 8.3 Hz, 1H, H5’), 6.89 (d, J = 2.1 Hz, 1H, H2’), 6.66 (dd, J = 8.3, 2.1 Hz, 1H, H6’), 5.03 (s, 2H, CH2), 4.47 (s, 1H, H4), 3.73 (s, 3H, OMe). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 160.5, 159.0, 151.0, 148.7, 146.5, 137.7, 137.5, 136.3, 132.3, 131.1, 129.4, 128.4, 121.9, 121.7, 119.9, 119.1, 115.3, 113.7, 112.0, 111.8, 109.6, 69.1, 57.7, 55.5, 36.2. Anal. calcd. for C26H18ClN3O3: C, 68.50; H, 3.98; N, 9.22. Found: C, 68.72; H, 4.10; N, 9.38.
2-Amino-4-(4-((4-bromobenzyl)oxy)-3-methoxyphenyl)-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (6n)
White solid; Mp: 255-256 °C; IR (KBr): υ (cm-1) = 3429, 3339, 2910, 2197, 1683. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.78 (s, 1H, NH), 7.91 (d, J = 8.1 Hz, 1H, H10), 7.61 – 7.55 (m, 3H,, H2’’, H6’’, H9), 7.39 (d, J = 8.4 Hz, 2H, H3’’, H5’’), 7.34 (d, J = 8.1 Hz, 1H, H7), 7.30 (t, J = 8.1 Hz, 1H, H8), 7.25 (s, 2H, NH2), 6.93 (d, J = 8.4 Hz, 1H, H5’), 6.90 (d, J = 2.1 Hz, 1H, H2’), 6.66 (dd, J = 8.4, 2.1 Hz, 1H, H6’), 5.01 (s, 2H, CH2), 4.48 (s, 1H, H4), 3.73 (s, 3H, OMe). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 161.1, 160.5, 159.0, 151.0, 148.7, 146.5, 137.7, 137.5, 136.7, 131.3, 131.1, 130.0, 129.7, 121.9, 121.7, 120.8, 119.9, 119.1, 115.3, 113.7, 112.0, 111.8, 109.6, 69.2, 57.7, 55.5, 36.2. Anal. calcd. for C26H18BrN3O3: C, 62.41; H, 3.63; N, 9.34. Found: C, 62.35; H, 3.69; N, 9.41.
2-Amino-4-(3-methoxy-4-((4-methylbenzyl)oxy)phenyl)-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (6o)
White solid; Mp: 208-209 °C; IR (KBr): υ (cm-1) = 3460, 3339, 3243, 2921, 2203, 1685. 1H NMR (400 MHz, DMSO-d6) δ (ppm): δ 11.80 (s, 1H, NH), 7.92 (d, J = 8.1 Hz, 1H, H10), 7.59 (t, J = 8.1 Hz, 1H, H9), 7.34 – 7.22 (m, 6H, H2’’, H6’’, H8, H7, NH2), 7.19 (d, J = 7.9 Hz, 2H, H3’’, H5’’), 6.94 (d, J = 8.3 Hz, 1H, H5’), 6.90 (d, J = 2.1 Hz, 1H, H2’), 6.67 (dd, J = 8.3, 2.1 Hz, 1H, H6’), 4.98 (s, 2H, CH2), 4.49 (s, 1H, H4), 3.73 (s, 3H, OMe), 2.30 (s, 3H, CH3). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 161.0, 159.5, 153.9, 151.5, 149.2, 147.3, 137.7, 137.5, 134.6, 133.4, 129.5, 129.4, 128.7, 128.3, 124.7, 122.2, 120.4, 119.6, 115.8, 114.0, 112.5, 110.2, 70.3, 58.3, 56.0, 36.7, 21.3. Anal. calcd. for C27H21N3O3: C, 72.47; H, 4.86; N, 9.65. Found: C, 72.33; H, 4.90; N, 9.82.
In Vitro AChE and BChE Inhibition Assay
AChE (E.C. 3.1.1.7, Type V-S, lyophilized powder from electric eel), BChE (E.C. 3.1.1.8 from equine serum), acetylthiocholine iodide (ATCI), butyrylthiocholine iodide (BTCI), and 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB) were purchased from Sigma-Aldrich Company. The solutions of the title compounds were prepared in a mixture of dimethyl sulfoxide (DMSO, 5 mL) and methanol (5 mL) and diluted in 0.1 M KH2PO4/K2HPO4 buffer (pH: 8.0) to obtain final assay concentrations. All experiments were achieved at 25 °C. Four different concentrations were tested for each compound in triplicate to obtain the range of 20%-80% inhibition for AChE and BChE. To measure in vitro AChE or BChE activities, modified Ellman’s method (22) was performed using a 96-well plate reader (BioTek ELx808). Each well contained 50 µL potassium phosphate buffer (KH2PO4/K2HPO4, 0.1 M, pH: 8), 25 µL of the sample was dissolved in 50% methanol and 50% DMSO and 25 µL enzyme (final concentration 0.22 U/mL in buffer). They were preincubated for 15 minutes at room temperature, and then 125 µL of DTNB (3 mM in buffer) was added. The hydrolysis of ATCI catalyzed by AChE was characterized spectrometrically at 405 nm, followed by the addition of the substrate (ATCI3 mM in water). The change in absorbance was measured at 405 nm after 20 minutes. The IC50 values were determined graphically from inhibition curves (log inhibitor concentration vs. percentage of inhibition). A control experiment was performed under the same conditions without the inhibitor, and the blank contained buffer, DMSO, DTNB, and the substrate. The described method was also used for the BChE inhibition assay (22).
Kinetic Studies of AChE Inhibition
For estimates of the inhibition model and inhibition constant Ki, the reciprocal plots of 1/V versus 1/[S] were obtained using different concentrations of the substrate. For this purpose, experiments were performed similar to enzyme inhibition assay (22). The rate of enzymatic reaction was obtained with different concentrations of the inhibitor and in the absence of the inhibitor. For each experiment, the reaction was started by adding substrate, and progress curves were recorded at 405 nm over 2 minutes. Next, double reciprocal plots (1/v vs. 1/[s]) were made using the slopes of progress curves to obtain the type of inhibition. The slopes of these reciprocal plots were then plotted against the concentration of the compound in a weighted analysis, and Ki was determined as the intercept on the negative x-axis. All rate measurements were performed in triplicate, and the data were analyzed by Microsoft Excel 2003.
Molecular Docking Study
Docking simulations were performed using AUTODOCK 4.2 software (http://autodock.scripps.edu/) (25). In this respect, the PDB structure of 6I0B was retrieved from the Brookhaven protein database (http://www.rcsb.org). Then, the water molecules and the inhibitor were removed, and the enzyme’s pdbqt was prepared by AutoDock Tools (version 1.5.6) using default parameters. The three-dimensional structure of compound 6l was prepared by MarvineSketch 5.8.3, 2012, and then ligand.pdbqt was provided by AutoDock Tools (version 1.5.6). The AutoDock scoring grid box was approximately fixed between the CAS and PAS (for AChE, x-center: 134.75, y-center: 112.63, z-center: 40.79). The grid size was set to 60 × 60 × 60 points with a spacing value of 0.375 Å. The prepared compound was docked to the AChE template using a Lamarckian genetic algorithm of an initial population of 150 randomly placed individuals, the maximum number of 2.5 × 106 energy evaluations, the maximum number of 27 000 generations, and the number of 100 GA runs. A cluster analysis was performed on the docking results using a root mean square tolerance of 2.0, and the lowest energy conformation of the highest populated cluster was selected for analysis. Graphic visualizations were performed by Discovery Studio client software (version 2021).
Results and Discussion
Chemistry
The routes to synthesize compounds 6a-o are shown in Scheme 1. For the synthesis of compounds 6a-o, a series of substituted benzyloxy benzaldehydes has been considered according to our previous work (23). In this stage, 4-hydroxy benzaldehyde, 3-hydroxy benzaldehyde, and 3-methoxy-4-hydroxy (1a-c) benzaldehyde were reacted with various benzyl halide derivatives (2) in the presence of K2CO3 as the base and dimethylformamide (DMF) as the solvent. Next, compounds 6a-o were obtained from the reaction of benzyloxy benzaldehydes (3a-o), malononitrile (4), and 4-hydroxyquinoline or 4-hydroxyquinoline-2(1H)-one (5) in the ethanol under reflux conditions at 80 °C in the presence of NH4OAc as a catalyst (24).
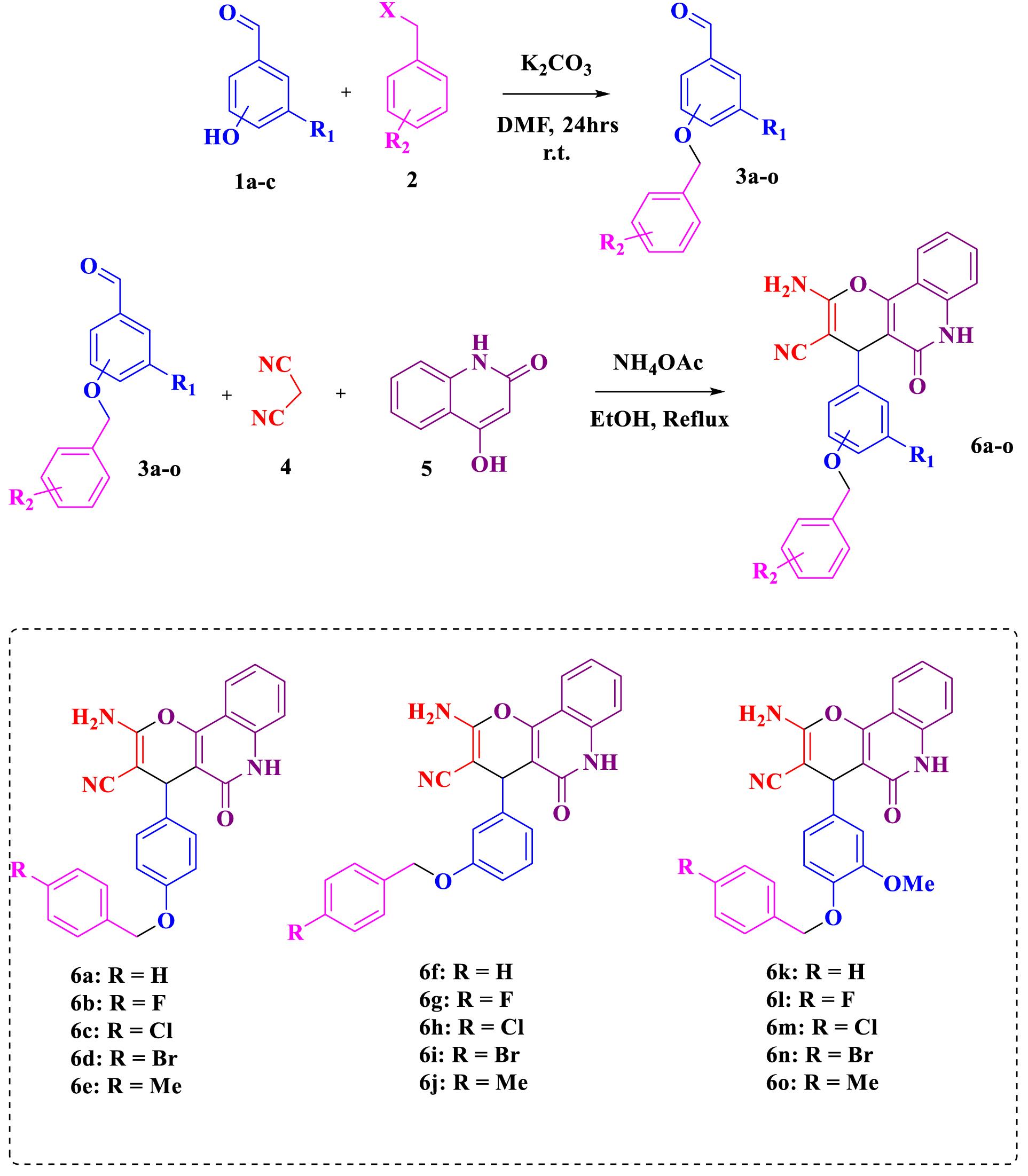
Scheme 1.
Synthesis of 2-Amino-pyrano[3,2-c]quinoline-3-carbonitrile Derivatives Bearing Benzyloxy Phenyl Moiety 6a-o
.
Synthesis of 2-Amino-pyrano[3,2-c]quinoline-3-carbonitrile Derivatives Bearing Benzyloxy Phenyl Moiety 6a-o
In Vitro Acetylcholinesterase and Butyrylcholinesterase Inhibition Assay
The in vitro ChE inhibitory activities of all synthesized compounds 6a-o were evaluated by modified Ellman’s method (22) and compared with donepezil as the reference drug (Table 1). The percentage of inhibition and half maximal inhibitory concentration (IC50) values were presented as the mean ± standard deviation (SD) of three independent experiments.
Table 1.
The IC50 Values of the Compounds 6a-o Against AChE and BChEa
|
Entry
|
Compound 6
|
AChE, Inhibition (%) 40 µg/L
|
BChE, Inhibition (%) 40 µg/L
|
BChEI, [IC50 (μM)]a
|
| 1 |
6a |
9.37 ± 1.06 |
30.76 ± 1.41 |
> 40 |
| 2 |
6b |
21.86 ± 2.79 |
21.45 ± 1.10 |
> 40 |
| 3 |
6c |
9.90 ± 0.83 |
48.63 ± 1.32 |
> 40 |
| 4 |
6d |
23.96 ± 0.69 |
26.51 ± 0.98 |
> 40 |
| 5 |
6e |
54.76 ± 1.34 |
74.59 ± 1.68 |
30.75 ± 0.67 |
| 6 |
6f |
31.67 ± 0.39 |
21.87 ± 0.86 |
> 40 |
| 7 |
6g |
7.48 ± 0.25 |
6.78 ± 0.16 |
> 40 |
| 8 |
6h |
2.61 ± 0.22 |
9.78 ± 0.24 |
> 40 |
| 9 |
6i |
36.92 ± 0.01 |
10.09 ± 1.27 |
> 40 |
| 10 |
6j |
21.93 ± 2.38 |
23.64 ± 0.89 |
> 40 |
| 11 |
6k |
36.71 ± 1.42 |
95.95 ± 1.21 |
1.08 ± 0.09 |
| 12 |
6l |
52.69 ± 0.32 |
98.98 ± 1.17 |
1.00 ± 0.07 |
| 13 |
6m |
49.37 ± 2.28 |
87.45 ± 1.29 |
2.63 ± 0.11 |
| 14 |
6n |
48.30 ± 0.99 |
79.26 ± 1.83 |
11.08 ± 0.03 |
| 15 |
6o |
18.07 ± 1.97 |
18.81 ± 1.53 |
4.23 ± 0.35 |
| 16 |
Donepezilb |
87.98 ± 0.01 |
91.06 ± 0.27 |
0.46 ± 0.03 |
Note. SD: Standard deviation; IC50: Half maximal inhibitory concentration; AChE: Acetylcholinesterase; BChE: Butyrylcholinesterase.
aInhibitor concentration (Mean ± SD of three experiments) required for 50% inactivation of AChE and BChE; bIC50 against AChE for donepezil = 0.035 ± 0.001 μM.
Based on the structure, the compounds can be divided into three series based on benzyloxy phenyl moiety, including 4-(benzyloxy) phenyl (6a-e), 3-(benzyloxy) phenyl (6f-j), and 4-(benzyloxy)-3-methoxyphenyl derivatives (6k-o). Based on the results (Table 1), all synthesized compounds had small activity against the AChE enzyme, and the results were expressed as a percentage of inhibition. Among the synthesized compounds, the compound of the third series (6k-o) represented good inhibition against the BChE enzyme. Moreover, the synthesized compounds of the first series 6a-e and the second series 6f-j had negligible activity against BChE. The compound 6e was the only active compound in the first series 6a-e. It seems that the presence of 3-methoxy in the middle ring of the molecule has an important rule for inhibitory activity against BChE. The docking studies also confirmed that the methoxy group could interact with the critical residue of the peripheral anionic site (PAS) of BChE.
The compound 6k without substitution and compound 6l with fluorine at the 4th position of the benzyl ring demonstrated the best anti-BChE activities with IC50 values of 1.00 ± 0.07 and 1.08 ± 0.09 μM, respectively. Introduction of the electron-withdrawing groups (EWGs), including fluorine, chlorine, and bromine, at the 4th position of the benzyl ring led to the production of compounds 6l, 6m, and 6n with IC50 values of 1.08 ± 0.09, 2.63 ± 0.11, and 11.08 ± 0.03 μM, respectively. These results indicated that the size of EWGs affects the anti-BChE activity so that with the increasing size of EWGs, inhibitory effects decrease and the orders of activities are 4-F > 4-Cl > 4-Br.
The introduction of 4-methyl as an electron-donating group on the benzyl ring created compound 6o with good BChE inhibitory activity (IC50 = 4.23 ± 0.35μM). Based on these results, electronic effects did not significantly contribute to the inhibitory activity, and probably the size of substitutions on the benzyl group had an important role in anti-BChE activities.
Kinetic Studies of Butyrylcholinesterase Inhibition
To determine the mechanism of inhibition for compound (6l) as the most potent anti-BChE agent, a linear regression analysis was performed by Lineweaver-Burk plot. Based on Figure 3, with increasing inhibitor concentration, Km increases whereas Vmax decreases compared to the values for the uninhibited reaction, indicating a mixed-type inhibition. As a result, compound 8g could bind to both enzyme and enzyme-substrate, but with different affinities. The estimate of the inhibition constant (Ki) for mixed-type inhibition is the same as the IC50 (22).
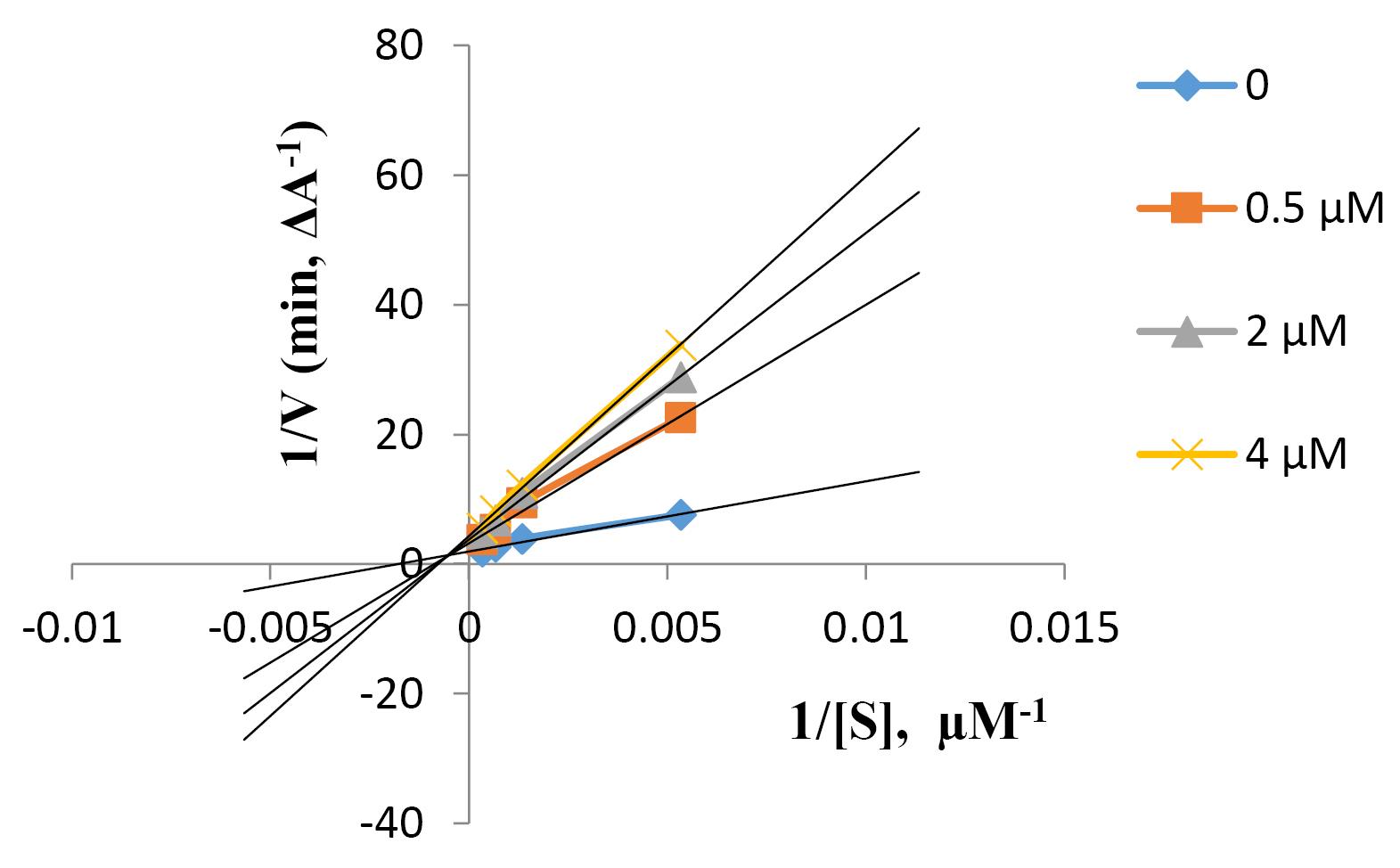
Figure 3.
Lineweaver-Burk Plot for the Inhibition of BChE by Compound 6l. Note. BChE: Butyrylcholinesterase
.
Lineweaver-Burk Plot for the Inhibition of BChE by Compound 6l. Note. BChE: Butyrylcholinesterase
Molecular Docking
To study the interactions of the most potent anti-BChE compound 6l in the active site of BChE, a computational study was performed using the AutoDock 4.2 package with Discovery Studio 4.0 Client. Since compound 6l has a chiral centre at the 4-position of the pyran ring, both (R)- and (S)-enantiomers were used for docking studies. The superposed structure of S-chlorotacrine-tryptophan as a ligand of crystallography and both enantiomers of compound 6l in the active site are illustrated in Figure 4. All structures were inserted into the active site of BChE as a U-shape so that two ends of molecules occupied the catalytic anionic site (CAS) and acyl binding pocket. In addition, the middle of them created interaction with the key amino acids of PAS (26).
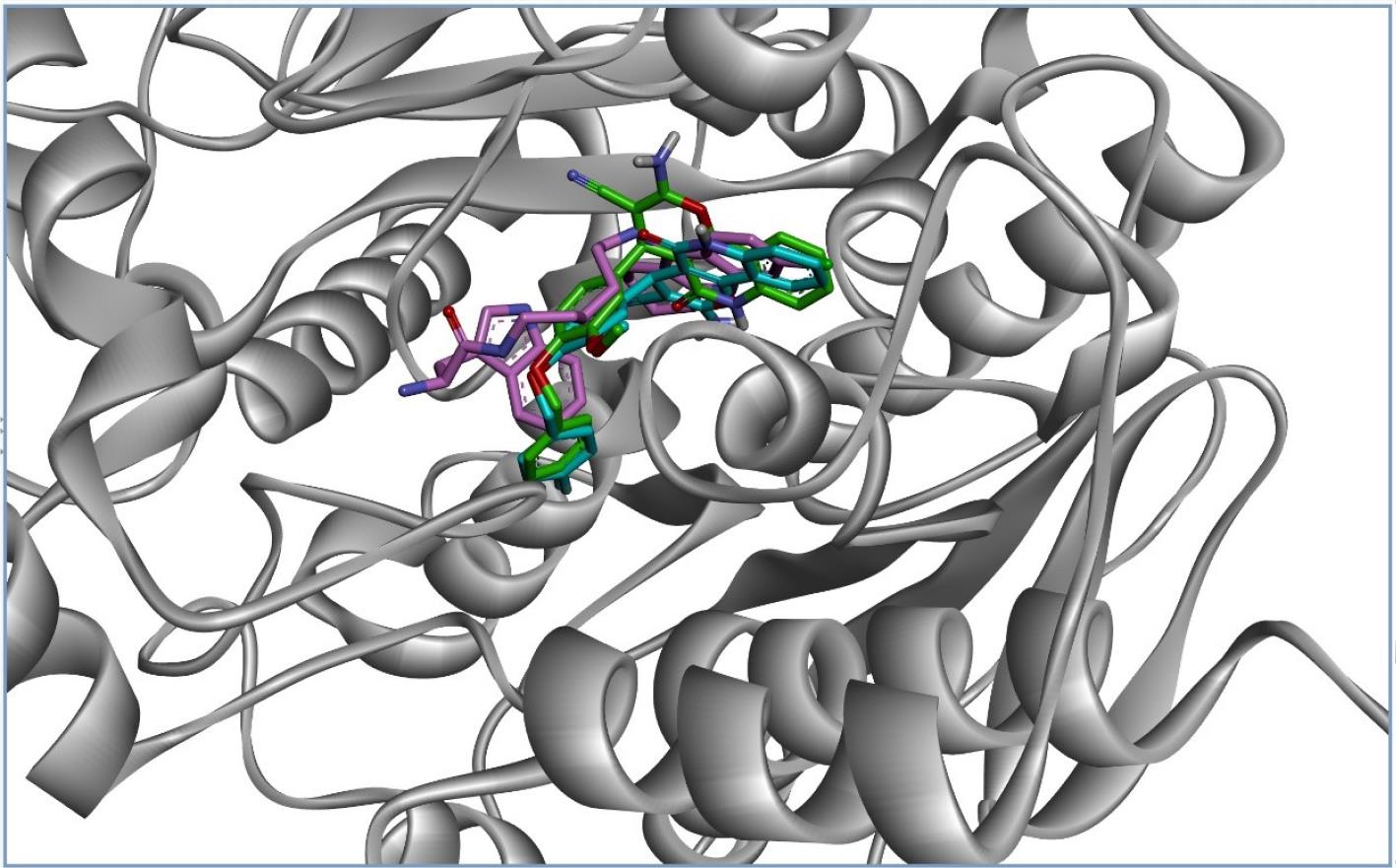
Figure 4.
The Superimposition of (R)-enantiomer (Blue) and (S)-enantiomer (Green) of the Compound 6l, and (S)-enantiomer of Chlorotacrine-tryptophan (Pink) as the Native Ligand of Crystallography in the Active Site of BChE Predicted by Molecular Docking. Note. BChE: Butyrylcholinesterase
.
The Superimposition of (R)-enantiomer (Blue) and (S)-enantiomer (Green) of the Compound 6l, and (S)-enantiomer of Chlorotacrine-tryptophan (Pink) as the Native Ligand of Crystallography in the Active Site of BChE Predicted by Molecular Docking. Note. BChE: Butyrylcholinesterase
According to Figure 5, the 2-amino-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile ring of (R)-enantiomer was oriented toward the CAS and quinolone moiety created π-π stacking with amino acids Trp82 and Trp430. The NH2 group made H-binding with Glu197 of the catalytic triad. The key amino acids Tyr332 and Phe329of PAS indicated hydrophobic interaction with OMe of the phenyl ring. The benzyl ring was directed to the acyl binding pocket of the enzyme and demonstrated alkyl-π and π-π interactions with Leu286 and Trp231, respectively.
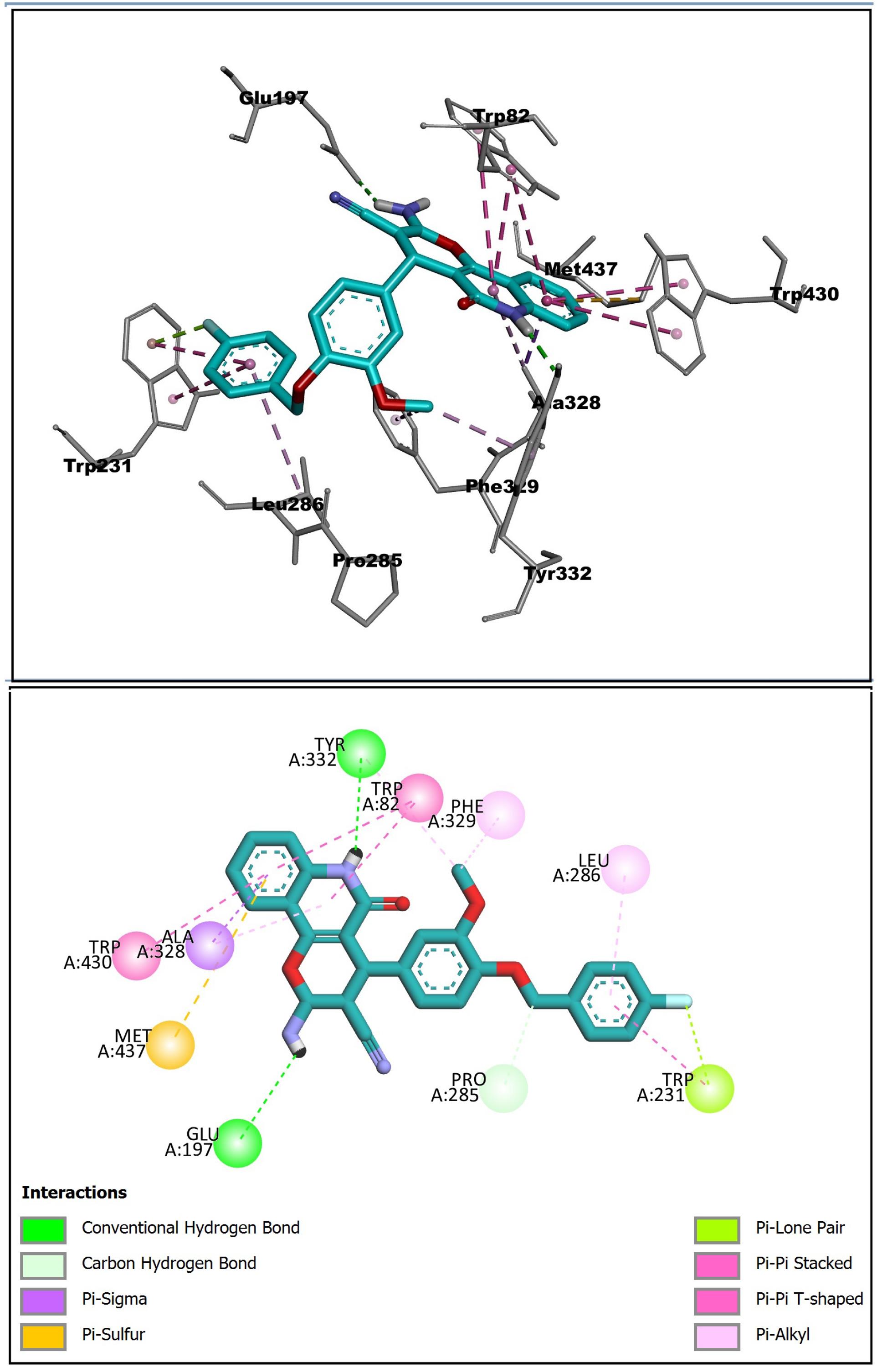
Figure 5.
Interaction of (R)-Enantiomer (cyano) of Compound 6l in the Active Site of BChE. Note. BChE: Butyrylcholinesterase. The hydrophobic and π-π interactions are displayed as purple dashed lines. Hydrogen bonds are shown by green dashed lines
.
Interaction of (R)-Enantiomer (cyano) of Compound 6l in the Active Site of BChE. Note. BChE: Butyrylcholinesterase. The hydrophobic and π-π interactions are displayed as purple dashed lines. Hydrogen bonds are shown by green dashed lines
As depicted in Figure 6, the 2-amino-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrilering of (S)-enantiomer was inversely oriented to CAS compared to the (R)- enantiomer of compound 6l, while another part of structure made interactions similar to the (S)-enantiomer. The quinolone ring showed π-π and π-alkyl interactions with amino acids Trp82, Tyr440, and Ala328 of CAS. The NH of quinolone indicated H-binding with His438 of the catalytic triad. The critical amino acids Tyr332, and Phe329 of PAS demonstrated a hydrophobic interaction with OMe of the middle phenyl ring and Asp70 showed H-binding with the 2-NH2 group. The benzyl ring was oriented to the acyl binding pocket of the enzyme and revealed alkyl-π and π-π interactions with Leu286 and Trp231, respectively.
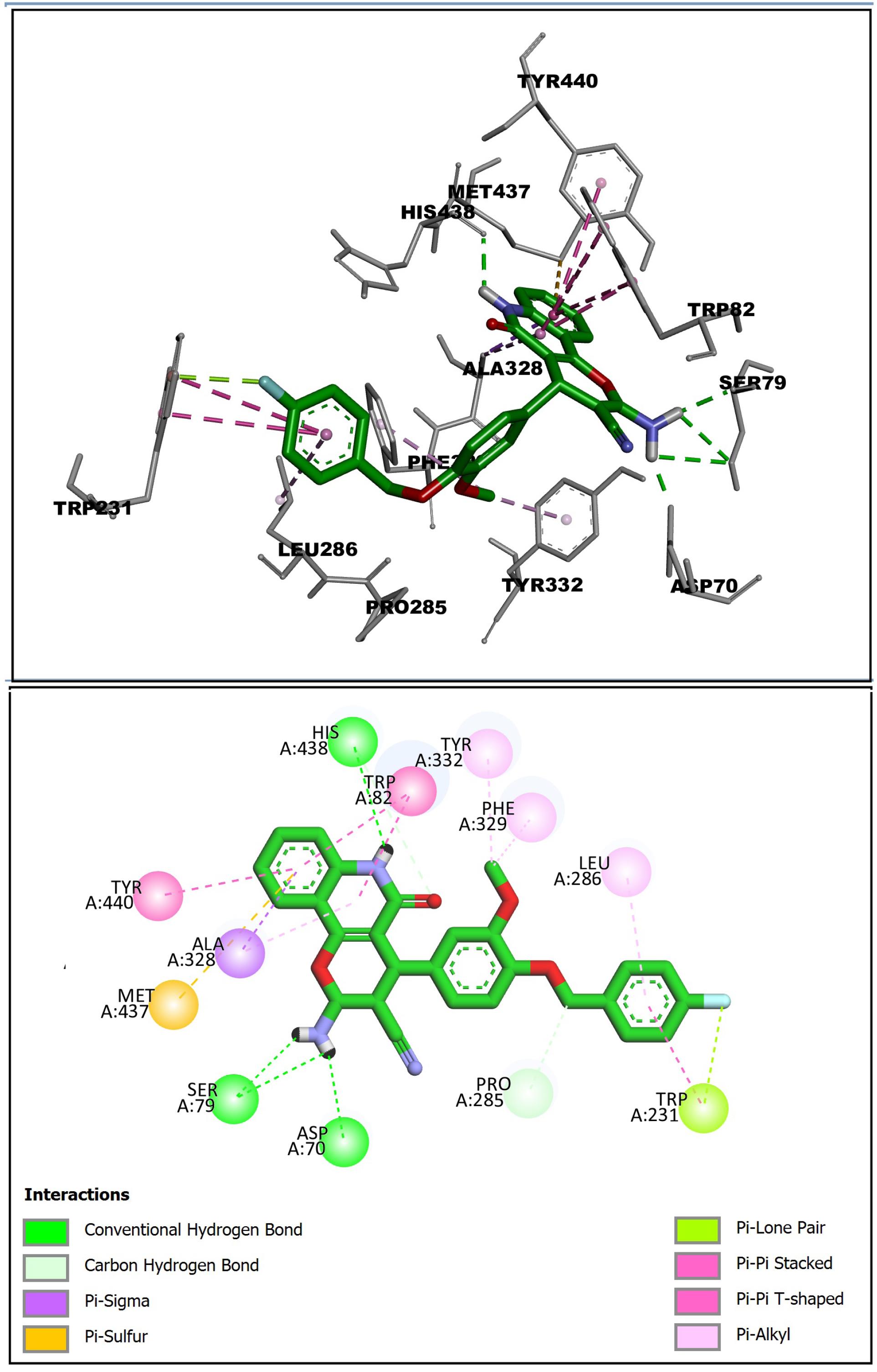
Figure 6.
Interaction of (S)-Enantiomer (Green) of Compound 6l in the Active Site of BChE. Note. BChE: Butyrylcholinesterase. Hydrogen bonds are depicted by green dashed lines. The hydrophobic and π-π interactions are displayed as purple dashed lines
.
Interaction of (S)-Enantiomer (Green) of Compound 6l in the Active Site of BChE. Note. BChE: Butyrylcholinesterase. Hydrogen bonds are depicted by green dashed lines. The hydrophobic and π-π interactions are displayed as purple dashed lines
Conclusion
A new series of 2-amino-pyrano[3,2-c]quinoline-3-carbonitrile derivatives bearing benzyloxy phenyl moiety were designed, synthesized, characterized, and evaluated against AChE and BChE as potential agents for the treatment of AD. The synthesized compounds 6a-o are divided into three series based on benzyloxy phenyl moiety, namely, 4-(benzyloxy) phenyl (6a-e), 3-(benzyloxy) phenyl (6f-j), and 4-(benzyloxy)-3-methoxyphenyl derivatives (6k-o). The in vitro results showed that all synthesized compounds had small activity against AChE. However, the compound of the third series (6k-o) demonstrated significant inhibition against the BChE. The compound 6l represented the best anti-BChE activity with an IC50 value of 1.00 ± 0.07. The kinetic and molecular docking studies confirmed that 6l is a mixed inhibitor and binds to both the CAS and PAS of BChE. Further, in silico studies indicated that the methoxy group on the middle phenyl ring has a significant role in interacting with the PAS of the enzyme.
Acknowledgements
This work was supported by the Vice-chancellor for Research and Technology of Hamadan University of Medical Sciences with project No. 9908065475.
Authors’ Contribution
Conceptualization: Zahra Najafi.
Data curation: Gholamabbas Chehardoli, Fatemeh Karimi, Roshanak Hariri, Tahmineh Akbarzadeh.
Formal analysis: Gholamabbas Chehardoli, Fatemeh Karimi, Roshanak Hariri, Tahmineh Akbarzadeh.
Funding acquisition: Zahra Najafi.
Investigation: Fatemeh Karimi.
Methodology: Zahra Najafi.
Project administration: Zahra Najafi.
Resources: Zahra Najafi.
Software: Zahra Najafi.
Supervision: Zahra Najafi.
Validation: Zahra Najafi.
Visualization: Zahra Najafi.
Writing–original draft: Gholamabbas Chehardoli, Fatemeh Karimi, Zahra Najafi.
Writing–review & editing: Gholamabbas Chehardoli, Fatemeh Karimi, Zahra Najafi.
Competing Interests
Authors declare that there is no conflict of interests.
Data Availability Statement
The data that supports the results of this research are accessible in the supplementary material of this article.
Supplementary Files
Supplementary file contains Figures S1-S45.
(pdf)
References
- Dekkers W, Rikkert MO. What is a genetic cause? The example of Alzheimer’s disease. Med Health Care Philos 2006; 9(3):273-84. doi: 10.1007/s11019-006-9005-7 [Crossref] [ Google Scholar]
- Drachman DA. The amyloid hypothesis, time to move on: amyloid is the downstream result, not cause, of Alzheimer’s disease. Alzheimers Dement 2014; 10(3):372-80. doi: 10.1016/j.jalz.2013.11.003 [Crossref] [ Google Scholar]
- Pannuzzo M. Beta-amyloid pore linked to controlled calcium influx into the cell: a new paradigm for Alzheimer’s disease. Alzheimers Dement 2022; 18(1):191-6. doi: 10.1002/alz.12373 [Crossref] [ Google Scholar]
- Pickett EK, Herrmann AG, McQueen J, Abt K, Dando O, Tulloch J, et al. Amyloid beta and tau cooperate to cause reversible behavioral and transcriptional deficits in a model of Alzheimer’s disease. Cell Rep 2019;29(11):3592-604.e5. 10.1016/j.celrep.2019.11.044.
- Hampel H, Mesulam MM, Cuello AC, Khachaturian AS, Vergallo A, Farlow MR. Revisiting the cholinergic hypothesis in Alzheimer’s disease: emerging evidence from translational and clinical research. J Prev Alzheimers Dis 2019; 6(1):2-15. doi: 10.14283/jpad.2018.43 [Crossref] [ Google Scholar]
- Braak H, Del Tredici K. Spreading of tau pathology in sporadic Alzheimer’s disease along cortico-cortical top-down connections. Cereb Cortex 2018; 28(9):3372-84. doi: 10.1093/cercor/bhy152 [Crossref] [ Google Scholar]
- Bell SM, Barnes K, De Marco M, Shaw PJ, Ferraiuolo L, Blackburn DJ. Mitochondrial dysfunction in Alzheimer’s disease: a biomarker of the future?. Biomedicines 2021; 9(1):63. doi: 10.3390/biomedicines9010063 [Crossref] [ Google Scholar]
- Perez Ortiz JM, Swerdlow RH. Mitochondrial dysfunction in Alzheimer’s disease: role in pathogenesis and novel therapeutic opportunities. Br J Pharmacol 2019; 176(18):3489-507. doi: 10.1111/bph.14585 [Crossref] [ Google Scholar]
- Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (N Y) 2018; 4:575-90. doi: 10.1016/j.trci.2018.06.014 [Crossref] [ Google Scholar]
- Vellom DC, Radić Z, Li Y, Pickering NA, Camp S, Taylor P. Amino acid residues controlling acetylcholinesterase and butyrylcholinesterase specificity. Biochemistry 1993; 32(1):12-7. doi: 10.1021/bi00052a003 [Crossref] [ Google Scholar]
- Greig NH, Lahiri DK, Sambamurti K. Butyrylcholinesterase: an important new target in Alzheimer’s disease therapy. Int Psychogeriatr 2002; 14 Suppl 1:77-91. doi: 10.1017/s1041610203008676 [Crossref] [ Google Scholar]
- Meunier B. Hybrid molecules with a dual mode of action: dream or reality?. Acc Chem Res 2008; 41(1):69-77. doi: 10.1021/ar7000843 [Crossref] [ Google Scholar]
- Grover P, Bhardwaj M, Mehta L, Kapoor G, Chawla PA. Current developments in the pyran-based analogues as anticancer agents. Anticancer Agents Med Chem 2022; 22(19):3239-68. doi: 10.2174/1871520621666211119090302 [Crossref] [ Google Scholar]
- Borah B, Dwivedi KD, Chowhan LR. Review on synthesis and medicinal application of dihydropyrano[3,2-b]pyrans and spiro-pyrano[3,2-b]pyrans by employing the reactivity of 5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one. Polycycl Aromat Compd 2022; 42(9):5893-937. doi: 10.1080/10406638.2021.1962923 [Crossref] [ Google Scholar]
- Yadav P, Shah K. Quinolines, a perpetual, multipurpose scaffold in medicinal chemistry. Bioorg Chem 2021; 109:104639. doi: 10.1016/j.bioorg.2021.104639 [Crossref] [ Google Scholar]
- Najafi Z, Mahdavi M, Saeedi M, Karimpour-Razkenari E, Asatouri R, Vafadarnejad F. Novel tacrine-1,2,3-triazole hybrids: in vitro, in vivo biological evaluation and docking study of cholinesterase inhibitors. Eur J Med Chem 2017; 125:1200-12. doi: 10.1016/j.ejmech.2016.11.008 [Crossref] [ Google Scholar]
- Khoobi M, Alipour M, Sakhteman A, Nadri H, Moradi A, Ghandi M. Design, synthesis, biological evaluation and docking study of 5-oxo-4,5-dihydropyrano[3,2-c]chromene derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors. Eur J Med Chem 2013; 68:260-9. doi: 10.1016/j.ejmech.2013.07.038 [Crossref] [ Google Scholar]
- Zaib S, Munir R, Younas MT, Kausar N, Ibrar A, Aqsa S. Hybrid quinoline-thiosemicarbazone therapeutics as a new treatment opportunity for Alzheimer’s disease‒synthesis, in vitro cholinesterase inhibitory potential and computational modeling analysis. Molecules 2021; 26(21):6573. doi: 10.3390/molecules26216573 [Crossref] [ Google Scholar]
- Babaee S, Chehardoli G, Akbarzadeh T, Zolfigol MA, Mahdavi M, Rastegari A. Design, synthesis, and molecular docking of some novel tacrine based cyclopentapyranopyridine‐and tetrahydropyranoquinoline‐kojic acid derivatives as anti‐acetylcholinesterase agents. Chem Biodivers 2021; 18(6):e2000924. doi: 10.1002/cbdv.202000924 [Crossref] [ Google Scholar]
- Shaikh S, Pavale G, Dhavan P, Singh P, Uparkar J, Vaidya SP. Design, synthesis and evaluation of dihydropyranoindole derivatives as potential cholinesterase inhibitors against Alzheimer’s disease. Bioorg Chem 2021; 110:104770. doi: 10.1016/j.bioorg.2021.104770 [Crossref] [ Google Scholar]
- Mo J, Yang H, Chen T, Li Q, Lin H, Feng F. Design, synthesis, biological evaluation, and molecular modeling studies of quinoline-ferulic acid hybrids as cholinesterase inhibitors. Bioorg Chem 2019; 93:103310. doi: 10.1016/j.bioorg.2019.103310 [Crossref] [ Google Scholar]
- Najafi Z, Alaei M, Bahmani A, Akbarzadeh T, Hariri R, Chehardoli G, Fused 1,4‐Dihydropyridines and Their Corresponding Pyridines: Synthesis, Molecular Modeling and Cholinesterase Inhibition. ChemistrySelect 2023; 8: 202300219. 10.1002/slct.202300219.
- Somakala K, Amir M, Sharma V, Wakode S. Synthesis and pharmacological evaluation of pyrazole derivatives containing sulfonamide moiety. Monatsh Chem 2016; 147(11):2017-29. doi: 10.1007/s00706-016-1694-x [Crossref] [ Google Scholar]
- Elinson MN, Ryzhkov FV, Nasybullin RF, Vereshchagin AN, Egorov MP. Fast efficient and general PASE approach to medicinally relevant 4H,5H-pyrano-[4,3-b]pyran-5-one and 4,6-dihydro-5H-pyrano-[3,2-c]pyridine-5-one scaffolds. Helv Chim Acta 2016; 99(9):724-31. doi: 10.1002/hlca.201600150 [Crossref] [ Google Scholar]
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 2009; 30(16):2785-91. doi: 10.1002/jcc.21256 [Crossref] [ Google Scholar]
- Macdonald IR, Martin E, Rosenberry TL, Darvesh S. Probing the peripheral site of human butyrylcholinesterase. Biochemistry 2012; 51(36):7046-53. doi: 10.1021/bi300955k [Crossref] [ Google Scholar]