Avicenna Journal of Pharmaceutical Research. :1-7.
doi: 10.34172/ajpr.2022.1066
Original Article
Patterns of Intravenous Human Immunoglobulin Administration in a Middle Eastern Teaching Hospital
Yasmin Zabihi 1  , Maryam Etminaniesfahani 2, Maryam Rangchian 2, *
, Maryam Etminaniesfahani 2, Maryam Rangchian 2, * 
Author information:
1Pharmacy School, Hamadan University of Medical Sciences, Hamadan, Iran
2Department of Clinical Pharmacy, Pharmacy School, Hamadan University of Medical Sciences, Hamadan, Iran
Abstract
Background: Drug use evaluation (DUE) helps to investigate and modify the pattern of drug administration with the aim of improving patient care and cost saving. Considering the important indications for intravenous immunoglobulin (IVIG) and its high cost, assessment of its prescription pattern could be helpful in increasing the efficiency of the health system.This study aimed to investigate the pattern of IVIG use in a tertiary teaching hospital.
Methods: This retrospective study included all inpatients who received IVIG in spring and summer 2020. The needed information was extracted from patients’ files. Data were analyzed using SPSS and compared with the standard guidelines.
Results: A total of 72 patients received IVIG. The indications were "FDA-approved" and "CEDITacknowledged" in 33.3% and 61.1% of the cases, respectively, and 45.8% adhered to the "red" indications of the UK protocol. Moreover, all prescriptions were in accordance with the approved indications of the FDO (Iranian Food and Drug Organization) guideline. Guillain-Barré syndrome (GBS), chronic inflammatory demyelinating polyneuropathy, and COVID-19 were the three most common causes of IVIG administration. Additionally, 66.7% had received the recommended dose regimen and 51.3% experienced drug side effects requiring some measures.
Conclusion: The occurrence of adverse drug reactions in more than half of the studied patients and related costs substantiate the need for enhancing physicians’ refrain from the unnecessary prescription of the IVIG, nursing staff’s knowledge, and the inclusion of a clinical pharmacist in the healthcare team.
Keywords: IVIG, Immune globulin, DUE, Drug use evaluation, Rational prescription
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Zabihi Y, Etminaniesfahani M, Rangchian M. Patterns of intravenous human immunoglobulin administration in a Middle Eastern teaching hospital. Avicenna J Pharm Res. 2022; 3(1):1-7. doi:10.34172/ajpr.2022.1066
Introduction
Intravenous immunoglobulin (IVIG) is a costly biological product extracted from healthy donated plasma. The final product contains antibodies of all donors and includes 5-8 g/dL of protein, 90% of which is IgG (1). This product was introduced in 1980 to manage immunodeficiency, and by creating an appropriate concentration of antibodies, it has caused the removal of inflammation induced by a broad spectrum of pathogens in immunodeficient patients. In addition, it modulates the immune system in the treatment of autoimmune diseases. Today, its application has expanded to medical fields such as neurology, hematology, dermatology, nephrology, and rheumatology (2,3).
Irrational medicine prescription is a worldwide issue (4). Optimizing medication utilization not only reduces drug costs but also improves patients’ outcomes regarding lower side effects and higher effectiveness (5). Drug use evaluation (DUE) is an effort to investigate the pattern of drug administration and, if necessary, modify it based on the standard guidelines (4). Studying the pattern of IVIG administration and utilization is considered an important research topic due to its important role in the control and treatment of many diseases, the increasing number of its indications and irrational prescriptions, the limited information on its proper use (especially in the Middle East), its high cost, and ultimately, scarce resources of preparation (2,6).
In recent years, the number of off-label indications for IVIG has expanded, but there is insufficient clinical evidence for many of them. The disease stage, severity, optimal circumstances, and the availability of less expensive substitutions with fewer side effects should also be considered (6,7). Despite the effectiveness and good tolerance in many cases, there are reports regarding the occurrence of serious side effects that must be considered (1). Many countries have addressed this issue and various policies have been devised to monitor and regulate the IVIG administration (8). In this regard, this study aimed to evaluate IVIG utilization pattern in an educational hospital and compare it with international guidelines.
Materials and Methods
This descriptive cross-sectional study was conducted with a retrospective design at Shahid-Beheshti Teaching Hospital in western Iran, affiliated with Hamadan University of Medical Sciences. In order to evaluate the pattern of IVIG prescription and utilization, all inpatients who received IVIG during a 6-month period (spring and summer 2020) were included in the study. The relevant data, such as demographic information (age, gender, and weight), laboratory and clinical parameters, admission ward, diagnosis, medication dosage, length of treatment, and side effects of medicines were obtained by reviewing patients’ records. The compliance of the IVIG prescription with the guidelines developed by the FDA (United States Food and Drug Administration), the CEDIT (Committee for the Evaluation and Diffusion of Innovative Technologies), the FDO (Iranian Food and Drug Organization) and the UK protocol was also examined. SPSS was used for data analysis.
Based on the FDA guideline, the IVIG indications are subcategorized into three main categories: FDA-approved, Off-labeled with support (strong evidence showing its effectiveness), and Off-labeled without support (there is no evidence to substantiate its use) (6,7). In the CEDIT standard guideline, IVIG indications are classified into three subgroups: acknowledged, under assessment, and unwarranted (9). Considering the UK protocol, the indications are graded using four colors: (a) red: IVIG therapy is highly evidence-based and considered critical (the highest priority), (b) blue: the use of IVIG is based on reasonable evidence and should be modified when there is a shortage of this drug (moderate priority), (c) gray: IVIG treatment is based on weak evidence and should be planned for each case (the latest priority), and (d) black: the use of IVIG is not recommended (10). Finally, the FDO guideline lists the indications for the IVIG that is considered approved by this organization (11).
Results
Table 1 summarizes the included cases’ demographics and details of IVIG prescriptions. As shown in Table 1, out of 72 patients included in the study, 34 cases (47.2%) were male. The IVIG was prescribed for 15 different indications, described in detail in Table 1. The cases’ age ranged from 19 years to 90 years, with a mean of 46.1 years, of whom 8 (11.1%) were elderly (65 years of age or older). Additionally, 17 patients (23.6%) were affected by diabetes mellitus, for whom some precautions had to be taken. Hydration was performed by infusion of 500 mL of 0.3% sodium chloride in 13.9% of IVIG recipients. Besides, 37 cases (51.38%), including 22 women and 15 men, experienced side effects after IVIG infusion. Moreover, 14 patients suffered from only one complication, while 23 cases had more than one complication. Shortness of breath (dyspnea) (11.1%), bleeding at the injection site (9.7%), nausea and vomiting (8.3%), and infection (8.3%) were the four most frequent side effects. Among the patients experiencing side effects, 3 cases (4.1%) had a high initial infusion rate, which was adjusted by the physician.
Table 1.
Patients’ Demographics and Details of IVIG Prescriptions
|
Variable
|
Results
|
|
Patients’ demographics
|
|
| Age (year) |
19 – 90 |
| Gender (n) (%) |
34 (47.2) male and 38 (52.8) female |
| Weight (kg) |
35 - 100 |
|
Details of IVIG prescriptions
|
|
Hospital ward in which IVIG prescribed
|
(n)(g)
|
| Neurology |
(41) (4615) |
| Oncology / Hematology |
(10) (785) |
| Nephrology |
(7) (570) |
| Infectious disease |
(6) (410) |
| Respiratory |
(5) (155) |
| Rheumatology |
(3) (350) |
|
Cause of IVIG administration
|
(n) (%) (g)
|
| Guillain-Barre syndrome (GBS) |
(18) (25) (2340) |
| Chronic inflammatory demyelinating polyneuropathy |
(CIDP) (11) (15.3) (1055) |
| COVID-19 |
(10) (13.9) (605) |
| Bronchiectasis due to secondary acquired hypogammaglobulinemia |
(4) (5.6) (80) |
| Idiopathic thrombocytopenic purpura (ITP) |
(4) (5.6) (340) |
| Kidney transplantation rejection |
(4) (5.6) (420) |
| Multifocal motor neuropathy (MMN) |
(MMN) (4) (5.6) (425) |
| Systemic lupus erythematosus (SLE) |
(4) (5.6) (295) |
| Autoimmune encephalitis |
(3) (4.2) (350) |
| Polymyositis |
(3) (4.2) (265) |
| Myasthenia gravis |
(2) (2.8) (180) |
| Multiple sclerosis (MS) |
(2) (2.8) (225) |
| Disseminated intravascular coagulation (DIC) associated with systemic vasculitis |
(1) (1.4) (60) |
| Chronic lymphocytic leukemia (CLL) |
(1) (1.4) (120) |
| Adult-onset Still’s disease (AOSD) |
(1) (1.4) (125) |
| Consumed IVIG (g) |
6,885 |
|
Adherence to FDA guideline
|
(n) (%) / (g)
|
| FDA approved |
(24) (33.3) / (2020) |
| Off-Labeled with support |
(32) (44.4) / (3780) |
| Off-Labeled without support |
(6) (8.4) / (480) |
| COVID-19 |
(10) (13.9) / (605) |
|
Adherence to UK guideline
|
(n) (%) / (g)
|
| Red |
(33) (45.8) / (3735) |
| Blue |
(18) (25) / (1490) |
| Gray |
(8) (11.1) / (705) |
| Black |
(3) (4.2) / (350) |
| COVID-19 |
(10) (13.9) / (605) |
|
Adherence to CEDIT guideline
|
(n) (%) / (g)
|
| Acknowledged |
(44) (61.1) / (4540) |
| Under assessment |
(12) (16.7) / (1220) |
| Unwarranted |
(6) (8.3) / (520) |
| COVID-19 |
(10) (13.9) / (605) |
|
Adherence to FDO guideline
|
(n) (%) / (g)
|
| Yes |
(62) (86.1) / (6280) |
| No |
(-) (0) |
| COVID-19 |
(10) (13.9) / (605) |
|
Adverse drug reactions
|
(n) (%)
|
| Shortness of breath (dyspnea) |
(8) (11.1) |
| Bleeding at the injection site |
(7) (9.7) |
| Infection/nausea and vomiting |
(6) (8.3) |
| Allergic reactions/constipation/fever and chills/hypotension/skin rashes |
(5) (6.9) |
| Itching/weakness |
(4) (5.6) |
| Headache/loss of consciousness/shock/urinary retention |
(3) (4.2) |
| Thrombosis |
(2) (2.8) |
| Bradycardia/chest pain/cough/increased heart rate/increased serum creatinine/meningitis/night sweats/seizures/stomach pain/swallowing disorders (dysphagia) |
(1) (1.4) |
IVIG: intravenous immunoglobulin, FDA: food and drug administration, UK: United Kingdom, CEDIT: committee for the evaluation and diffusion of innovative technologies, FDO: Iranian food and drug administration
Based on the records, clinical and laboratory parameters of each patient had been assessed before IVIG infusions, and renal function was continuously monitored. Unfortunately, 10 patients expired during the therapy, 4 of whom were infected with COVID-19. The remaining 62 patients were discharged after treatment completion.
The most common reason for prescribing IVIG was Guillain-Barré syndrome (GBS) (25%), which adhered to the “off-labeled with support” category of the FDA guideline, the “acknowledged” subgroup of the CEDIT guideline, “red” indications of the UK protocol, and approved groups of the FDO protocol.
A total of 1377 vials of IVIG (6885 g) were administered during this study period, of which 388 vials (2020 g) and 908 vials (4540 g) were consumed for the indications approved by the FDA and the CEDIT guidelines, and 747 vials (3735 g) were utilized for the “red” indications of the UK protocol. Moreover, except for the amount used to treat COVID-19 (605 g), the amount of IVIG administration (6280 g) for all indications was approved by the FDO guideline (Table 1).
Considering IVIG administration based on the ward, the majority (4,615 g, 67%) of IVIG prescriptions belonged to the neurology ward. The number of patients and the amount of administered IVIG in each ward have been summarized in Table 1. Regarding the treatment length, the minimum and maximum lengths were 1 and 5 days, with a median of 4 days.
In terms of dosage, the standards state that the dose of IVIG should be calculated based on the patient’s body weight. In the present study, the weight of the patients varied from 35 kg to 100 kg, with a mean of 66.47 kg. In 48 patients (66.7%), the prescribed dose was in accordance with guidelines, 5 cases (6.9%) received a dose higher than the recommended one, and 7 cases (9.7%) experienced an underprescribing. It might be worth mentioning that in the case of 12 patients, guidelines have not determined any recommended dose for IVIG, because the effectiveness of this medicine in these cases has not been proven. Additionally, 10 patients (13.9%) were infected with coronavirus, 1 case had adult-onset Still’s disease (AOSD), and 1 case was a pregnant woman with vaginal bleeding and a history of spontaneous miscarriage.
The 15 observed indications for IVIG administration in this study were categorized based on the FDA guideline, the CEDIT guideline, and the UK protocol (Figures 1, 2, 3 and Table 1). Regarding the FDO guideline, the IVIG prescription was approved for all cases.
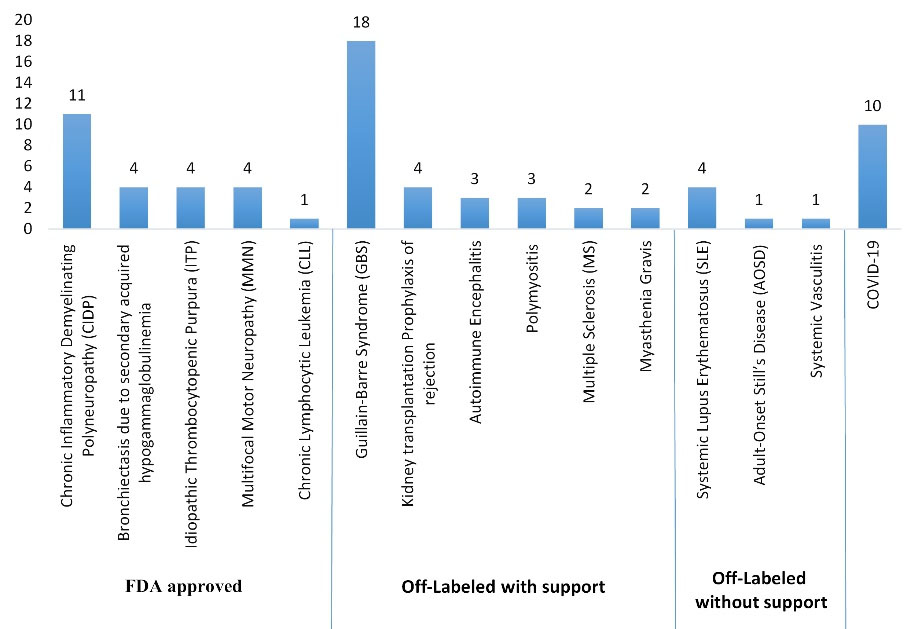
Figure 1.
Indications for IVIG Administration Based on the FDA Guideline
.
Indications for IVIG Administration Based on the FDA Guideline
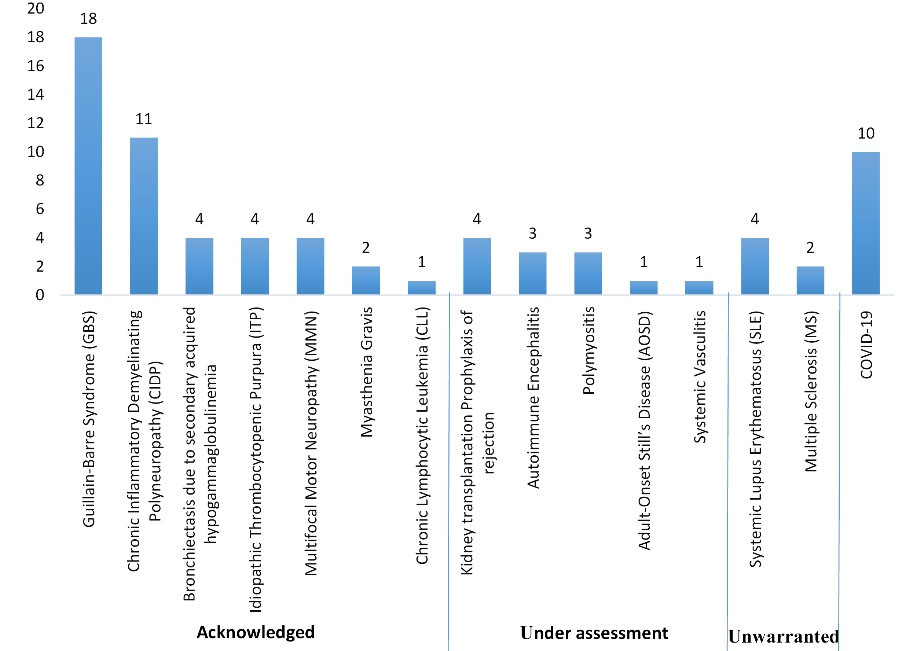
Figure 2.
Indications for IVIG Administration Based on the CEDIT Guideline
.
Indications for IVIG Administration Based on the CEDIT Guideline
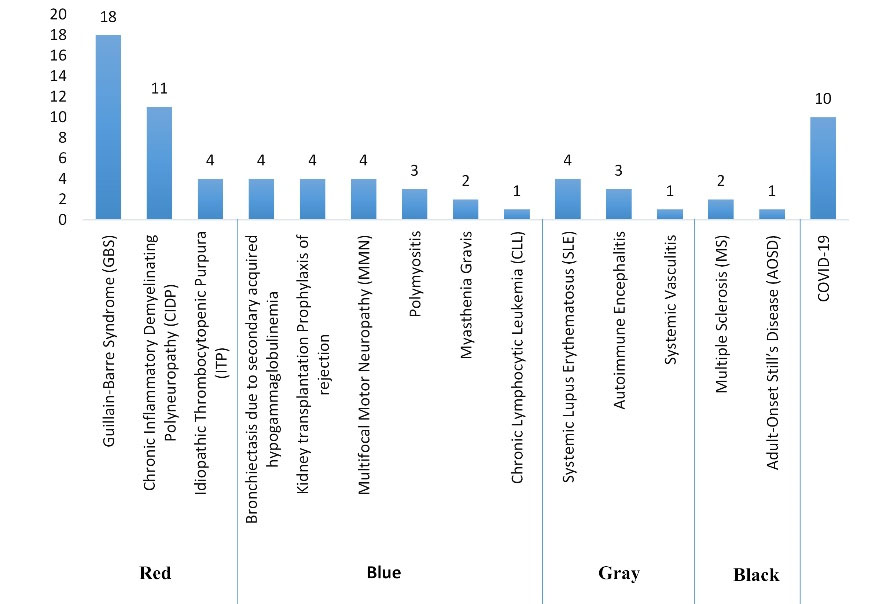
Figure 3.
Indications for IVIG Administration Based on the UK Guideline
.
Indications for IVIG Administration Based on the UK Guideline
Considering the FDA guideline, the highest percentage of IVIG use was assigned to the “off-labeled with support” indications (44.4% of patients). The percentage of IVIG administration for the “FDA-approved” and “off-labeled without support” indications was 33.3% and 8.4%, respectively. Based on the CEDIT guideline, 61.1% of the indications were assigned to the “acknowledged” subgroup, and the percentage of IVIG administration for “under assessment” and “unwarranted” indications of this guideline was 16.7% and 8.3%, respectively. Considering the UK protocol, the prescription of IVIG adhered to the “red,” “blue,” “gray,” and “black” indications in 45.8%, 25%, 11.1%, and 4.2% of the cases, respectively. Additionally, 10 of the 72 cases (13.9%) were infected with coronavirus and received IVIG as supportive care. However, during our study period, there was not any recommended dose regimen in the guidelines, and this indication was not included in any of the mentioned categories.
Discussion
IVIG is a costly blood product employed for the treatment of various pathological conditions by raising antibody concentrations (1,2). Several probable mechanisms for the effects of this product have been identified, but the relative contribution of each mechanism to the various disorders remains uncertain (1). Considering the limited access to IVIG around the world, increasing unlicensed usage, high production costs, possible adverse reactions, and inadequate information on its effectiveness, especially in the Middle East, physicians need to take special precautions while using this drug properly. Evaluation of IVIG misuse has been one of the priorities of healthcare providers for the past several years (6,12,13). DUE studies can help to investigate the pattern of drug consumption and, if required, modify it based on standard guidelines (5). The findings of DUE can help physicians to improve the quality of their prescriptions, which in turn enables policy-makers in the health care systems to make better decisions and plans.
The FDA has approved the use of IVIG for the following purposes: treatment of primary immunodeficiency, prevention of bacterial infections in secondary immunodeficient individuals with hypogammaglobulinemia and chronic lymphocytic leukemia (CLL), prevention of coronary artery aneurysms in Kawasaki disease, increase of platelets counts in idiopathic thrombocytopenic purpura (ITP) to prevent or control bleeding, reduction of serious bacterial infection in HIV-infected children, prevention of infections and pneumonitis in acute graft-versus-host disease, chronic inflammatory demyelinating polyneuropathy (CIDP), and multifocal motor neuropathy (MMN) (6). Many organizations and nations have IVIG administration protocols. The current research examined the pattern of IVIG consumption in a teaching hospital and assessed its adherence to four guidelines belonged to the FDA, CEDIT, UK, and FDO guidelines.
In our study, 18 hospitalized patients (or 25%) received IVIG for GBS. The remaining indications were as follows: CIDP (15.3%), COVID-19 (13.9%), bronchiectasis due to secondary acquired hypogammaglobulinemia, ITP, kidney transplantation rejection, systemic lupus erythematosus (SLE), and MMN (with the same percentage of 5.6%), polymyositis and autoimmune encephalitis (AE) (each 4.2%), multiple sclerosis (MS) and myasthenia gravis (MG) (each 2.8%), and ultimately, CLL, AOSD, and systemic vasculitis (with the same percentage of 1.4%). The frequency of FDA-approved indications such as CIDP, ITP, MMN, CLL, and bronchiectasis due to secondary acquired hypogammaglobulinemia was 33.3% in our study, which is comparable to a similar study conducted on IVIG consumption at King Khalid University Hospital over a 3-year period. In that study, 305 patients were identified, and 109 of them (35.7%) received IVIG for FDA-approved indications (13).
Speaking about adherence to the guidelines, 61.1% of IVIG indications were assigned to the “acknowledged” subgroup of the CEDIT guideline, which agrees with what Frauger et al discovered in another study conducted in three French university hospitals in 2006. In other words, 70% of IVIG indications were assigned to the “acknowledged” subgroup of the CEDIT guideline, and 9% and 18% of IVIG prescriptions were for “under assessment” and “unwarranted” indications, respectively (9). It has been reported in previous studies that despite the availability of comprehensive guidelines, physicians sometimes prescribe this product in situations where it has not been recommended or as a last resort when conventional treatments have failed. Overall, more than half of IVIG prescriptions worldwide were for indications that are not approved by the FDA. Among them are hemolytic disorders of neonates who do not respond to phototherapy, prevention of the incidence of nosocomial infections in neonates born weighing less than 2500 grams, AIDS-related thrombocytopenia, multiple myeloma associated with hypogammaglobulinemia, autoimmune hemolytic anemia, GBS, MS, MG, solid organ transplantation, dermatomyositis, necrotizing fasciitis, and so on. Patterns of IVIG utilization continue to change (14). It is worth noting that during our six-month observation period in 2020, the spread of the coronavirus was a significant issue; in fact, 13.9% of all hospitalized patients were infected with this virus and received IVIG. The multifunctional long-term effects of immunoglobulin therapy make it a suitable therapeutic candidate for patients hospitalized with COVID-19. After GBS and CIDP, COVID-19 was the third most frequent indication for IVIG. However, there was no recommendation on the dose, timing, or rate of IVIG infusion in our employed guidelines during the study period.
Considering the adverse drug reactions, in a study by Björkander et al, conducted on 49 patients receiving IVIG, only 4.7% of them experienced adverse drug reactions related to IVIG infusion (15). Ruiz-Antorán et al studied systemic reactions to IVIG in 13 Spanish hospitals. A total of 119 patients (21.4%) experienced infusion-related adverse effects. In 28 (5%) of them, this complication was serious, and in 96 patients (17%), the infusion was stopped or modified (16). In contrast, more than half of the IVIG patients in our study (37 of them, or 51.38%) experienced adverse drug reactions, which is significantly more than what was reported in earlier studies. More than half of the patients (51.4%) who indicated adverse drug reactions had IVIG infusions for “off-label with support” indications, 32.4% had IVIG infusions for the “FDA-approved” indications, and 2.7% had infusions for “off-label without support” indications. Additionally, 10.8% of the patients who experienced infusion-related adverse effects were infected with COVID-19. In the case of the CEDIT guideline, 67.6%, 18.9%, and 2.7% of patients with complications belonged to the “acknowledged”, “under assessment”, and “unwarranted” indications, respectively. Based on the UK protocol, 51.4%, 27%, 5.4%, and 5.4% of patients with reported complications followed the “red”, “blue”, “gray”, and “black” indications, respectively.
Based on the literature, the majority of adverse drug reactions are mild and are consequences of a high infusion rate, infection, brand change, the patient’s sensitivity, and the initial infusion (17,18). In Iran, IVIG is not accessible in a generic form, and a commercial product may not always be available. Obviously, it is vital to follow the manufacturer’s suggested infusion instructions. By performing premedication, taking the right precautions, and infusing IVIG at a rational rate, the possibility of adverse drug effects will be greatly reduced (19). Patients with a history of renal failure, patients who take nephrotoxic medicines, patients over 65 years old, patients with diabetes, and cardiovascular patients should be hydrated prior to receiving an infusion (20). In the study conducted by Brennan, 41% of adverse drug reactions happened in patients who needed to take pre-infusion measures. Staff and patients who administer IVIG infusions need to be evaluated and educated regularly to lower the risk of side effects (19).
When it comes to the rational medication, the appropriate dosing should also be addressed. The dose of IVIG should be calculated based on the patient’s body weight. In our study, more than half of the cases (66.7%) received the recommended dose, 5 cases (6.9%) received more, and 7 cases (9.7%) received less. In October 2010, pharmacist-led research at Brigham and Women’s Hospital focused on IVIG dosing per ideal body weight for each indication. There were a total of 418 patients. For 2.2% and 0.5% of them, doses and administration frequencies were not in compliance with the related guideline (21). A cross-sectional study performed in a teaching hospital located in the east of Iran reported that about half of the cases had received the recommended dose regimen, around one-third (28.6%) received a dose less than the recommended one, and 2 cases (4.1%) received an excess dose (22).
GBS was the most common IVIG prescription in this research. Plasmapheresis may be used in addition to IVIG to relieve the patient’s symptoms. Plasma exchange and IVIG infusion are equally effective treatments for GBS. However, IVIG is substantially more expensive (23). Plasmapheresis and corticosteroid therapy are two other therapy choices for the treatment of CIDP, which was the second most common indication for IVIG prescription. Each of the three treatment approaches has both advantages and disadvantages, and their efficacy is comparable (24).
Our research had some limitations, including a small sample size and insufficient patient medical records, such as infusion length, in the patients’ files.
Conclusion
It was revealed that, in the studied patients, IVIG was widely utilized for “off-labeled” and “unwarranted” indications. The lack of evidence on the effectiveness of IVIG in many situations, the high cost of production, and the limited procurement resources all substantiate the necessity for regular follow-ups. Practitioners should administer this medicine with more caution and avoid prescribing it inappropriately. Overprescription of a medicine might cause negative health effects, higher treatment expenses, and treatment failure. One of the main aims of drug utilization evaluation is to cut down on direct and indirect drug costs caused by irrational prescriptions. Most Iranian hospitals suffer from a lack of human resources. Participation of the pharmacy department in medicine preparation and the inclusion of a clinical pharmacist in the healthcare team can improve treatment outcomes, prevent avoidable adverse drug reactions, and reduce treatment costs for patients and society. In this research, for the first time, two European guidelines and the FDO guideline were applied along with the FDA guideline. Based on the findings, it is recommended that interventional trials on IVIG be conducted to provide the evidence needed to develop regional and national recommendations, as well as studies to find strategies for better control of the related costs.
Acknowledgements
The authors would like to appreciate Shaihd-Beheshti Hospital for the cooperation, as well as the Research Deputy of Hamadan University of Medical Sciences.
Authors’ Contribution
Conceptualization: Maryam Etminani.
Data curation: Yasmin Zabihi, Maryam Rangchian.
Formal analysis: Maryam Rangchian.
Methodology: Maryam Etminani.
Project administration: Maryam Etminani, Maryam Rangchian.
Writing – original draft: Yasmin zabihi.
Writing – review & editing: Maryam Rangchian, Maryam Etminani.
Competing Interests
The authors declare that they have no conflict of interests.
Ethical Approval
Ethics Committee of Hamadan University of Medical Sciences examined and approved the study protocol (ID: IR.UMSHA.REC.1399.602).
Funding
This article was elicited from the first author’s Pharm.D thesis and was supported by Hamadan University of Medical Sciences (grant number: 9908065470).
References
- Looney RJ, Huggins J. Use of intravenous immunoglobulin G (IVIG). Best Pract Res Clin Haematol 2006; 19(1):3-25. doi: 10.1016/j.beha.2005.01.032 [Crossref] [ Google Scholar]
- Lucas M, Lee M, Lortan J, Lopez-Granados E, Misbah S, Chapel H. Infection outcomes in patients with common variable immunodeficiency disorders: relationship to immunoglobulin therapy over 22 years. J Allergy Clin Immunol 2010;125(6):1354-60.e4. 10.1016/j.jaci.2010.02.040.
- Jolles S, Sewell WA, Misbah SA. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol 2005; 142(1):1-11. doi: 10.1111/j.1365-2249.2005.02834.x [Crossref] [ Google Scholar]
- Phillips MS, Gayman JE, Todd MW. ASHP guidelines on medication-use evaluation American Society of Health-system Pharmacists. Am J Health Syst Pharm 1996; 53(16):1953-5. doi: 10.1093/ajhp/53.16.1953 [Crossref] [ Google Scholar]
- WHO Drug and Therapeutics Committee Training Course - Participant’s Guide. Available from: https://apps.who.int/iris/handle/10665/68553. Accessed 2007.
- Perez EE, Orange JS, Bonilla F, Chinen J, Chinn IK, Dorsey M. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol 2017; 139(3S):S1-S46. doi: 10.1016/j.jaci.2016.09.023 [Crossref] [ Google Scholar]
- Yang L, Wu EY, Tarrant TK. Immune gamma globulin therapeutic indications in immune deficiency and autoimmunity. Curr Allergy Asthma Rep 2016; 16(8):55. doi: 10.1007/s11882-016-0632-7 [Crossref] [ Google Scholar]
- Rocchio MA, Schurr JW, Hussey AP, Szumita PM. Intravenous immune globulin stewardship program at a tertiary academic medical center. Ann Pharmacother 2017; 51(2):135-9. doi: 10.1177/1060028016673071 [Crossref] [ Google Scholar]
- Frauger E, Grassi J, Pradel V, Bornet C, Rouby F, Delorme J. Use of intravenous immunoglobulins in clinical practice: data from three French university hospitals. Fundam Clin Pharmacol 2011; 25(6):753-61. doi: 10.1111/j.1472-8206.2010.00908.x [Crossref] [ Google Scholar]
- The Clinical Guidelines for Immunoglobulin Use, Implemented in 2008. Gov.UK. Available from: https://www.gov.uk/government/publications/clinical-guidelines-for-immunoglobulin-use-second-edition-update. Accessed August 20, 2011.
- IVIG Prescription Guide from the Iranian Food and Drug Organization. Available from: http://treatment.sbmu.ac.ir/uploads/4-ivig_final.pdf. Accessed 2020.
- Fakhari Z, Farsaei S, Sabzghabaee AM. Predicting factors for the pattern of intravenous immunoglobulin utilization in a Middle Eastern University Hospital. J Res Pharm Pract 2018; 7(4):188-94. doi: 10.4103/jrpp.JRPP_18_73 [Crossref] [ Google Scholar]
- Alangari AA, Abutaleb MH, Albarraq AA, Al-Dhowailie AA. Intravenous immunoglobulin utilization in a tertiary care teaching hospital in Saudi Arabia. Saudi Med J 2008; 29(7):975-9. [ Google Scholar]
- Darabi K, Abdel-Wahab O, Dzik WH. Current usage of intravenous immune globulin and the rationale behind it: the Massachusetts General Hospital data and a review of the literature. Transfusion 2006; 46(5):741-53. doi: 10.1111/j.1537-2995.2006.00792.x [Crossref] [ Google Scholar]
- Björkander J, Wadsworth C, Hanson LA. 1040 prophylactic infusions with an unmodified intravenous immunoglobulin product causing few side-effects in patients with antibody deficiency syndromes. Infection 1985; 13(3):102-10. doi: 10.1007/bf01642867 [Crossref] [ Google Scholar]
- Ruiz-Antorán B, Agustí Escasany A, Vallano Ferraz A, Danés Carreras I, Riba N, Mateu Escudero S. Use of non-specific intravenous human immunoglobulins in Spanish hospitals; need for a hospital protocol. Eur J Clin Pharmacol 2010; 66(6):633-41. doi: 10.1007/s00228-010-0800-y [Crossref] [ Google Scholar]
- Dashti-Khavidaki S, Aghamohammadi A, Farshadi F, Movahedi M, Parvaneh N, Pouladi N. Adverse reactions of prophylactic intravenous immunoglobulin; a 13-year experience with 3004 infusions in Iranian patients with primary immunodeficiency diseases. J Investig Allergol Clin Immunol 2009; 19(2):139-45. [ Google Scholar]
- Kahwaji J, Barker E, Pepkowitz S, Klapper E, Villicana R, Peng A. Acute hemolysis after high-dose intravenous immunoglobulin therapy in highly HLA sensitized patients. Clin J Am Soc Nephrol 2009; 4(12):1993-7. doi: 10.2215/cjn.04540709 [Crossref] [ Google Scholar]
- Brennan VM, Salomé-Bentley NJ, Chapel HM. Prospective audit of adverse reactions occurring in 459 primary antibody-deficient patients receiving intravenous immunoglobulin. Clin Exp Immunol 2003; 133(2):247-51. doi: 10.1046/j.1365-2249.2003.02199.x [Crossref] [ Google Scholar]
- Winters JL, Brown D, Hazard E, Chainani A, Andrzejewski C, Jr Jr. Cost-minimization analysis of the direct costs of TPE and IVIg in the treatment of Guillain-Barré syndrome. BMC Health Serv Res 2011; 11:101. doi: 10.1186/1472-6963-11-101 [Crossref] [ Google Scholar]
- Rocchio MA, Schurr JW, Hussey AP, Szumita PM. Intravenous immune globulin stewardship program at a tertiary academic medical center. Ann Pharmacother 2017; 51(2):135-9. doi: 10.1177/1060028016673071 [Crossref] [ Google Scholar]
- Moradi M, Moti T. Drug use evaluation of human intravenous immunoglobulin (IVIG) in a teaching hospital in East of Iran. J Pharm Care 2016; 4(3-4):70-4. [ Google Scholar]
- Kozanoglu I, Deniz Y, Buyukkurt N, Yeral M, Boga C, Ozdogu H. A retrospective study on patients with Guillain-Barré syndrome treated with therapeutic plasma exchange and other treatment options–a centre’s experience. Eur Neurol Rev 2015; 10(1):81-4. [ Google Scholar]
- Oaklander AL, Lunn MP, Hughes RA, van Schaik IN, Frost C, Chalk CH. Treatments for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): an overview of systematic reviews. Cochrane Database Syst Rev 2017; 1(1):CD010369. doi: 10.1002/14651858.CD010369.pub2 [Crossref] [ Google Scholar]