Avicenna Journal of Pharmaceutical Research. :37-44.
doi: 10.34172/ajpr.2022.1064
Original Article
Functional Components and Biological Activities of Peucedanum chenur Mozaff as a Natural Spice From the Apiaceae Family
Omid Asgari 1, Dara Dastan 1, * 
Author information:
1Department of Pharmacognosy, School of Pharmacy, Medicinal Plants and Natural Products Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
Abstract
Background: Peucedanum chenur Mozaff has been used by local people as a spice and for the treatment of urinary infections. The chemical constituent and biological activities of the essential oil (EO) and the extract of P. chenur Mozaff were evaluated in this study.
Methods: The EO components were qualitatively and quantitatively identified by gas chromatography/ mass spectrometry (GC/MS) and gas chromatography/flame ionization detector. The phytochemical analyses, total flavonoids, and phenolic contents of the extract were determined, and then the phenolic compounds of the extract were quantified by reverse-phase high-performance liquid chromatography.
Results: Based on the results, 36 compounds were identified in the volatile oil, accounting for 94.7% of the total oil. The major components were α-pinene, limonene, γ-terpinene, β-pinene, and sabinene. The EO exhibited significant antibacterial activity on Bacillus cereus, Escherichia coli, Staphylococcus aureus, and Klebsiella pneumoniae, whereas it exerted no effect on Pseudomonas aeruginosa. The antioxidant activity of the volatile oil had a half maximal inhibitory concentration value of 122±2.1 (μg/mL). Rutin, caffeic acid, naringenin, apigenin, and quercetin (183.6, 132.8, 38.4, 13.1, and 3.5 mg/100 g of the plant, respectively) were quantified in the methanolic extract.
Conclusion: The various bioactive compounds and the antibacterial and antioxidant activities of P. chenur Mozaff confirmed the potential of this plant for use in the food, pharmaceutical, and cosmetic industries.
Keywords: Antibacterial, Caffeic acid, DPPH, Essential oil, Peucedanum chenur Mozaff, α-Pinene
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Asgari A, Dastan D. Functional components and biological activities of peucedanum chenur mozaff as a natural spice from the Apiaceae family. Avicenna J Pharm Res. 2022; 3(1):37-44. doi:10.34172/ajpr.2022.1064
Introduction
The genus Peucedanum with more than 120 species, belonging to the Apiaceae family, is widely found in various regions of Asia, Europe, Africa, and Iran (with 21 species). Previous studies have shown that plants in the genus Peucedanum are rich sources of different compounds such as essential oils (EOs), coumarins, flavonoids, diterpenes, phenolic acids, and glycosides. They possess different biological effects such as anticancer, antimicrobial, antioxidant, anti-obesity or bodyweight reducing, cardiopulmonary protection, antihyperlipidemic, and antityrosinase properties. Furthermore, the plants of this genus have been used in traditional medicine in many countries as diuretic, expectorant, sedative, and antimicrobial agents and for the treatment of various problems such as headache, sore throat, coughs, and respiratory, cardiovascular, and inflammatory diseases (1).
In “The Canon of Medicine” by Avicenna, the Peucedanum grande fruit was mentioned as an effective diuretic in the prevention and treatment of kidney stones (2). Moreover,Peucedanum pastinacifolium is known as an antihyperlipidemic vegetable among the local people in Iran (3). Today, one of the challenges facing human beings is the resistance of pathogenic microorganisms to current antibiotics, underlining the need for new antibiotics. In this regard, the search for natural products with antibacterial effects to develop new antibiotics with different mechanisms of action and fewer side effects can be an important step to overcome this problem (4). Studies have revealed that EOs have remarkable antibacterial activity against different bacterial strains. In addition, combining EOs with antibiotics to increase the antibacterial effect and reduce the effective dose of antibiotics is a method that has recently attracted the interest of researchers to achieve a new generation of herbal medicines. The synergistic effects of the EOs obtained from some Peucedanum species in combination with other antibiotics have been established thus far (5,6).
EOs are volatile, nonpolar, and generally aromatic compounds, which are found in various parts of plants. Due to their various volatile compounds, EOs produce various biological effects such as antiseptic (antiparasitic, antiviral, antibacterial, and antifungal), insecticidal, antioxidant, and cytotoxic effects; demonstrating great potential uses in a variety of fields such as food, pharmaceutical, and cosmetic industries (e.g., as preservatives and flavors). The history of the application of EOs goes back to the Middle Ages (7,8).
The overactivity of free radicals in the body can lead to various injuries and diseases such as premature aging, cancer, and cardiovascular disorders. Antioxidants have been identified as important agents in the regeneration of damaged cells and the defense against oxidative stress. Functionally, there are different types of antioxidants, among which antioxidants that inhibit free radicals are considered one of the most essential antioxidants, and the study of their capacity has been the subject of much scientific research (9-11).
Peucedanum chenur Mozaff is one of the endemic plants of Iran that has not been studied so far. With regard to studies on the other species of the genus Peucedanum and their various pharmacological effects, in this study, the chemical composition and the antibacterial and antioxidant activities of P. chenurMozaff EOs were qualitatively and quantitatively investigated for the first time. Moreover, the plant extracts were phytochemically studied for the presence of different compounds. Concerning the presence of flavonoids and phenolic compounds in the other species of the genus Peucedanum, the total flavonoid and phenolic contents of the P. chenur Mozaff extract were evaluated in this investigation. Subsequently, the phenolic compounds of the methanolic extract were quantified by the high-performance liquid chromatography-photodiode array detector (HPLC-PDA).
Materials and Methods
Plant Materials
Peucedanum chenur Mozaff was collected from the natural habitat (Kurdistan province, Iran) 35.88993°N, 46.97429°E in the flowering season. The voucher specimen (Herbarium No. 409) of the plant was deposited in the Herbarium of the School of Pharmacy, Hamadan, Iran.
Preparation of EO
The volatile oil of the aerial parts of P. chenur Mozaff was obtained by the hydrodistillation technique for 4 hours. A sample (110 g) of the plant was ground and then added to a 2-L balloon flask, and mixed with 1 L of distilled water. The balloon flask was attached to the Clevenger apparatus under the heating mantle. The condenser of the Clevenger apparatus was insulated with cotton and aluminum foil to increase working efficiency. Finally, the volatile oil was dehydrated by anhydrous sodium sulfate and stored at 6°C.
Analysis of the Volatile Oil
The constituents of the EO were qualitatively and quantitatively identified by gas chromatography/mass spectrometry-flame ionization detector (GC/MS-FID; ThermoQuest Trace, UK). The fused-silica capillary column used for analysis was a capillary column (DB-5, 60 m, J & W Scientific®). The temperatures of the column were programmed from 60°C to 250°C (rate of 5 °C/minute), and the holding time was set at the 250°C for 10 minutes. The flow rate of the nitrogen gas as a mobile phase was 1 mL/min in the GC/FID, and the temperature of the injector and the detector was 250°C and 300°C, respectively. In the GC/MS analysis, the flow rate of the mobile phase (helium gas) was 1 mL/min, and the temperature of the interface and ion source was 240°C and 205°C, respectively. Moreover, the ionization energy was 70 eV, and the scan range for mass was 45-456 M/Z. Different parameters such as retention time, retention indices, MS spectra, and GC/MS libraries (Figure 1) were used to identify the constituents (12).
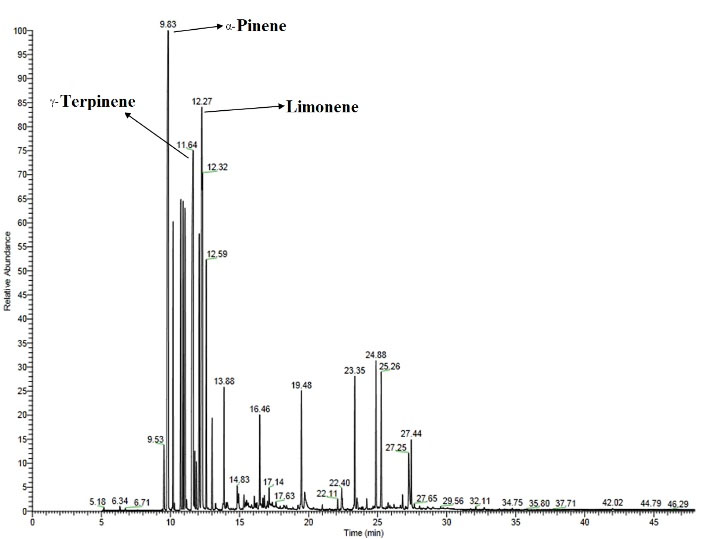
Figure 1.
GC‐MS Chromatogram of Peucedanum chenur Mozaff Essential Oil (Aerial Parts). Note. GC-MS: Gas chromatography-mass spectrometry
.
GC‐MS Chromatogram of Peucedanum chenur Mozaff Essential Oil (Aerial Parts). Note. GC-MS: Gas chromatography-mass spectrometry
Preparation of the Extracts
For the preparation of the extract by the maceration method, 100 g of the P. chenur Mozaff were ground and then soaked in 0.5 L of chloroform with shaking at room temperature for three days. The obtained extract was concentrated using a rotary evaporator at 50°C. This procedure was performed three times. Furthermore, the methanolic extract was obtained by the same procedure. The obtained extracts were evaporated and stored at 4°C.
Antibacterial Activity
The antibacterial activity of P. chenur Mozaff volatile oils was evaluated against the standard strains of bacteria (6 gram-positive and 3 gram-negative bacteria) by comparison with standard antibiotics (tetracycline, gentamicin, and ampicillin) using the disc diffusion method by measuring the inhibitory zone in the agar Mueller-Hinton medium and by minimum inhibitory concentration determination using the broth microdilution assay according to the method of Abdali et al. All analyses were performed in triplicates (13). The standard bacteria included Bacillus pumilus (PTCC 1274), Bacillus subtilis (ATCC 465), Staphylococcus aureus (ATCC 25923), Bacillus cereus (PTCC 1015), Klebsiella pneumoniae (ATCC 10031), Enterococcus faecalis (ATCC 29737), Escherichia coli (ATCC 25922), Staphylococcus epidermidis (ATCC 12228), and Pseudomonas aeruginosa (ATCC 85327).
Determination of Total Phenolic and Flavonoid Content
The total phenolic content was determined using the Folin-Ciocalteu method. Gallic acid was used as the standard, and the calibration curve was plotted after measuring the absorbance of different concentrations. A different concentration of the sample was prepared by dilution in methanol. In addition, 30 µL of the sample was mixed with 300 µL of the Folin-Ciocalteu reagent, and then, 200 µL of sodium carbonate was added to the solution, and finally, the absorbance was measured at 765 nm again.
The total flavonoid content of the extract was expressed as mg of quercetin equivalent per gram of the dry sample. A different concentration of the sample was provided by dilution in methanol. Next, 30 µL of the sample was mixed with 200 µL of sodium nitrite (1 M) and 200 µL of aluminum chloride. Eventually, after one hour, the absorbance was measured at 415 nm again. According to Quercetin as a standard reference, various concentrations were prepared, and the absorbance was recorded at 415 nm. All measurements were performed three times (14).
2,2-Diphenyl-1-picryl-hydrazyl-hydrate (DPPH) Radical Scavenging Activity
The antioxidant activity of the EO was assayed by the method of Dastan et al using ascorbic acid instead of BHT as the standard antioxidant. In this assay, various concentrations of ascorbic acid (1.25-250 µg/mL) were added to the DPPH solution (0.15 mM). The mixture was kept at room temperature for 60 minutes (a dark place with shaking), and then the absorbance was recorded at 517 nm (15).
Phytochemical Analysis
The initial phytochemical analyses of P. chenur Mozaff extracts were evaluated using standard methods employed by Ugochukwu et al (16) and Kumar Bargah (17).Further phytochemical analyses of P. chenur Mozaff extracts were evaluated using HPLC. The methanolic extract and standard compounds were analyzed using RP-HPLC-DAD equipped with a C18 reversed-phase column (25 cm × 4.6 mm, 3 µm) at 27°C. The flow rate was 1 mL/min, and the injection volumes were 20 μL of the sample. The applied method (water (A) and HPLC grade methanol (B)) included 0-10 minutes (30% B), 10-30 minutes (30-60% B), 30-55 minutes (60-100% B), and 55-65 minutes (100% B). The wavelength range of the device was also set from 200 to 400 nm. After the injection of the methanolic extract into the HPLC system, the extract chromatogram was compared with the existing standards, retention time, and both 2D and 3D UV spectra in order to identify the phenolic compounds. The amounts of the identified phenolic compounds in the extracts were determined using the calibration curve obtained from the standards. Finally, the amounts of compounds were expressed as µg/g of the dry extract, and ChemStation software was applied for data analysis.
Results and Discussion
The volatile oil obtained from P. chenur Mozaff was a thick and yellow liquid with a 0.5 (w/w) yield. The volatile oil components were identified and quantified by GC/MS-FID. A total of 94.7% of the EO components, including 36 different compounds, were identified (Table 1 and Figure 1). The main constituents of the EO were α-pinene (17.26), limonene (14.67), γ-terpinene (14.11), β-pinene (5.79), and sabinene (5.69), belonging to monoterpene hydrocarbons (Figure 2).
Table 1.
The Chemical Composition of the Essential Oil From the Aerial Parts of Peucedanum chenur Mozaff
|
No.
|
Compounds
|
Area% (±SDc) n=3
|
RIa
|
RIb
|
| 1 |
α‐thujene |
1.2 ± 0.1 |
920 |
924 |
| 2 |
α-pinened |
17.3 ± 1.2 |
932 |
932 |
| 3 |
Camphened |
4.0 ± 0.2 |
946 |
946 |
| 4 |
Sabinene |
5.7 ± 0.4 |
968 |
969 |
| 5 |
β‐pinene |
5.8 ± 0.5 |
974 |
974 |
| 6 |
β-myrcene |
4.8 ± 0.3 |
983 |
988 |
| 7 |
Dehydro-1,8-cineole |
0.1 ± 0.0 |
985 |
988 |
| 8 |
γ -terpinene |
14.1 ± 0.9 |
1001 |
1002 |
| 9 |
δ-3-carene |
0.7 ± 0.0 |
1005 |
1008 |
| 10 |
α-terpinene |
0.6 ± 0.0 |
1009 |
1014 |
| 11 |
ρ-cymene |
5.3 ± 0.4 |
1017 |
1020 |
| 12 |
Limonene |
14.7 ± 0.7 |
1023 |
1024 |
| 13 |
(z)-β-ocimene |
3.6 ± 0.2 |
1035 |
1032 |
| 14 |
γ-terpinene |
1.2 ± .01 |
1051 |
1054 |
| 15 |
cis-sabinene hydrate |
0.1 ± 0.0 |
1060 |
1065 |
| 16 |
Terpinolene |
1.8 ± 0.1 |
1082 |
1086 |
| 17 |
Linalool |
0.1 ± 0.0 |
1089 |
1095 |
| 18 |
cis-ρ-menth-2-en-1-ol |
0.4 ± 0.0 |
1115 |
1118 |
| 19 |
α-campholenal |
0.3 ± 0.0 |
1119 |
1122 |
| 20 |
cis-verbenol |
0.3 ± 0.0 |
1139 |
1137 |
| 21 |
trans-verbenol |
0.2 ± 0.0 |
1143 |
1140 |
| 22 |
pinocarvone |
0.3 ± 0.0 |
1159 |
1160 |
| 23 |
terpinen-4-ol |
1.6 ± 0.1 |
1173 |
1174 |
| 24 |
Cryptone |
0.2 ± 0.0 |
1181 |
1183 |
| 25 |
α-terpineol |
0.3 ± 0.0 |
1185 |
1186 |
| 26 |
Myrtenal |
0.1 ± 0.0 |
1193 |
1195 |
| 27 |
β-bourbonene |
0.5 ± 0.0 |
1392 |
1387 |
| 28 |
(E)-caryophyllene |
2.1 ± 0.1 |
1425 |
1417 |
| 29 |
γ-elemene |
0.3 ± 0.0 |
1435 |
1434 |
| 30 |
α-humulene |
0.2 ± 0.0 |
1458 |
1452 |
| 31 |
germacrene D |
2.1 ± 0.1 |
1490 |
1484 |
| 32 |
Bicyclogermacrene |
2.1 ± 0.2 |
1505 |
1500 |
| 33 |
δ-cadinene |
0.1 ± 0.0 |
1525 |
1522 |
| 34 |
Germacrene B |
0.2 ± 0.0 |
1564 |
1559 |
| 35 |
Spathulenol |
1.4 ± 0.1 |
1585 |
1577 |
| 36 |
Caryophyllene oxide |
1.1 ± 0.1 |
1593 |
1582 |
|
|
Monoterpene hydrocarbons |
80.9 |
|
|
|
|
Oxygenated monoterpenes |
3.8 |
|
|
|
|
Sesquiterpene hydrocarbons |
7.5 |
|
|
|
|
Oxygenated sesquiterpenes |
2.5 |
|
|
|
|
Total identified |
94.7 |
|
|
a Retention indices calculated from the homologous series C6‐C30; b Retention indices according to RI references; cStandard deviation for three replications; dThe identification was also confirmed by co-injection with standard.
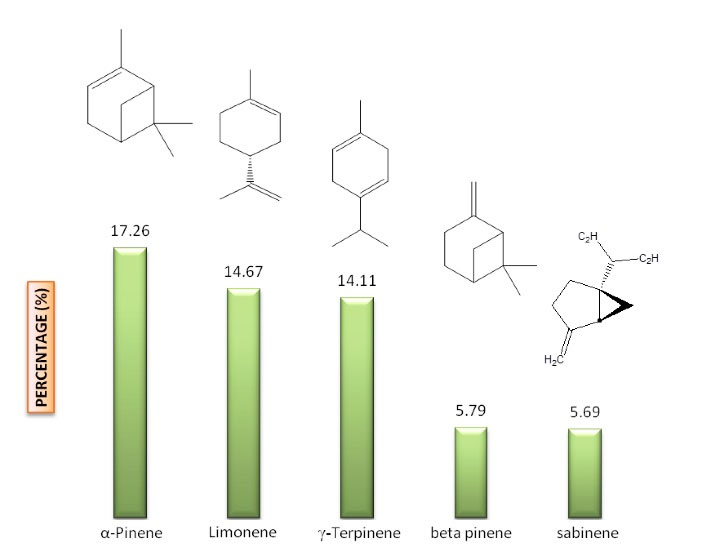
Figure 2.
The Main Compounds of the Peucedanum chenur Mozaff Essential Oils
.
The Main Compounds of the Peucedanum chenur Mozaff Essential Oils
Studies on the plants of the genus Peucedanum have also shown that the major constituents of the volatile oil of most species in this genus are monoterpene hydrocarbons, and in many species such as officinal, alsaticum, oreoselinum, austriacum, palimbioides, scoparium, and cervaria, the main compound is α-pinene (1).
Additionally, the quality and efficiency of the extraction of volatile oils vary in different studies, which can be affected by several factors such as the parts of the applied plant, environmental factors, harvest season, the genetic and intrinsic factors of the plant, the geographical location of the plant, plant storage conditions after collection, and the method used for the extraction of the EO. In a study performed by Masoudi et al on P. scoparium, the EO yield was 0.47% w/w (18). In another study on P. petiolare, Rustaiyan et al reported a volatile oil yield of 0.85% w/w (19). Studies on the other species of the genus Peucedanum also revealed the EO extraction yields from the aerial parts ranging from 0.02-0.64% v/w (1).
Based on the analysis of the volatile oil of P. austriacum, the volatile oil of the leaves differed from that of the fruit. The major constituents in the volatile oil of the leaves were sesquiterpenoids. Further, caryophyllene oxide (23.1%), germacrene D (12.2%), and (E)-caryophyllene (10.2%) were the major compounds. However, in the volatile oil obtained from the fruit, monoterpenoids formed the major part of the volatile oil, and β-phellandrene (45.2%) and α-pinene (10.1%) were the main compounds (20). Furthermore, in P. officinale, the efficiency and the number of the compounds in the volatile oils obtained from the flowers, stems, leaves, and roots of the plant were different, while monoterpenes were the main components in all parts of the plant (21).
According to studies on the EOs of the other Peucedanum species, α-pinene is the main compound in 7 species, including officinal, austriacum, oreoselinum, alsaticum, scoparium, palimbioides,andlongifolium, while limonene is the main compound in 4 species, including zenkeri, alsaticum, officinal,andcervaria. On the other hand, γ-terpinene is not the main compound in these 11 species. However, the results of these investigations can be used in the chemotaxonomy of the plants of the genus Peucedanum(1,19).
The most prominent feature of the EO of P. chenur Mozaff, compared with those of the other mentioned species, is the presence of γ-terpinene, along with limonene as the main constituents. Furthermore, the anti-tumor effects of limonene (22) and the anti-inflammatory effects of γ-terpinene (23) have been proven in previous studies. Hence, the P. chenur Mozaff plant can be considered in anti-tumor and anti-inflammatory studies, and further pharmacological studies on the EO of the plant should be conducted in this regard.
The results obtained from the initial phytochemical analyses of the P. chenur Mozaff extracts (Table 2) indicated the presence of different compounds such as steroids, tannins, alkaloids, phenols, flavonoids, terpenoids, and amino acids in this plant, which can be considered the source of pharmacological effects in the plant. Various compounds such as coumarins, amines, glycosides, flavonoids, phenolic acids, and diterpenes have been reported to exist in the Peucedanum species in previous studies. For example, the presence of flavonoids, diterpenes, saponins, alkaloids, and phenolic compounds in the plant has been detected in a phytochemical study conducted on P. beluchistanicum (24).
Table 2.
The Results of Phytochemical Analysis of the Peucedanum chenur MozaffExtract
|
Phytochemical Constituents
|
Methanol Extract
|
Chloroform Extract
|
| Flavonoids |
+++ |
- |
| Phenols |
+++ |
- |
| Alkaloids |
+ |
+ |
| Proteins |
- |
- |
| Terpenoids |
+ |
+ |
| Steroids |
+ |
+ |
| Saponins |
- |
- |
| Anthraquinones |
- |
- |
| Amino acids |
++ |
- |
Tannins
Glycoside |
++
- |
+
- |
Note. +++: Strongly present, ++: Moderately present; +: Slightly present; -: Absent; P. chenur: Peucedanum chenur.
The antioxidant capacity of the volatile oil of P. chenur Mozaff was determined by the DPPH free radical scavenging method, and a half maximal inhibitory concentration value of 122 ± 2.1 (μg/mL) was obtained accordingly. The amount of the total phenolic of the P. chenur Mozaff extract was 0.32 ± 0.07 mg of gallic acid equivalents per gram of the sample. Further, the amount of the total flavonoid for the P. chenur Mozaff extract was 0.97 ± 0.09 mg of quercetin equivalent per gram of the sample.
The application of plants containing phenolic compounds has been increasing in various food and pharmaceutical industries. The phenolic compounds in these plants have a variety of biological effects, including antioxidant, anti-cancer, and anti-cardiovascular disease effects (11). In a study performed by Tepe et al on the antioxidant capacity of P. longifolium and P. palimbioides using the beta-carotene-linoleic acid method, both species exhibited high antioxidant capacity (25).
The antibacterial activity results of the P. chenur Mozaff volatile oil are provided in Table 3. α-pinene and β-pinene are the major components of the P. chenur Mozaff volatile oil and exhibit considerable antibacterial effects, thus the volatile oil obtained from P. chenur Mozaff was expected to show antibacterial activities. The antibacterial test results demonstrated that the growth inhibition zone diameter for B. cereus and K. pneumoniae, as well as S. aureus and E. coli was 17 mm and 18 mm, respectively, indicating the significant antibacterial activity against these strains. Consequently, the volatile oil represented the greatest growth inhibitory effect on S. aureus and E. coli. However, the EO exhibited the weakest activity on P. aeruginosa. In the case of Gram-negative P. aeruginosa bacteria, it should be noted that gram-negative bacteria are more resistant species owing to their structural characteristics, thus the prevalence of resistant infections triggered by these bacteria has caused serious concern worldwide, especially P. aeruginosa, also known as multidrug-resistant bacteria in other studies (26).
Table 3.
In Vitro Antibacterial Activity of Peucedanum chenur Mozaff Essential Oil
|
Microorganisms
|
|
Sample
|
Bacillus
pumilus
|
Bacillus subtilis
|
Staphylococcus aureus
|
Bacillus cereus
|
Klebsiella
pneumoniae
|
Enterococcus
faecalis
|
Escherichia coli
|
Staphylococcus epidermidis
|
Pseudomonas aeruginosa
|
| Essential oil |
12a
(15)b |
12
( > 15) |
18
(7.5) |
17
(7.5) |
17
(7.5) |
12
(15) |
18
(7.5) |
12
( > 15) |
- |
| Tetracyclinec |
nt |
21
(3.2) |
20
(3.2) |
nt |
Nt |
Nt |
-
(nt) |
34
(1.6) |
Nt |
| Gentamicind |
nt |
-
(nt) |
-
(nt) |
nt |
Nt |
Nt |
23
(3.2) |
-
(nt) |
Nt |
| Ampicilline |
15
(15) |
14
(15) |
13
(15) |
nt |
Nt |
Nt |
12
(15) |
19
(15) |
Nt |
a Zone of inhibition (in mm) includes diameter of the disc (6 mm), b Minimum inhibitory concentration values as mg/mL, (-): Inactive, (7-13): Moderately active, ( > 14): Highly active; nt: Not tested, c Tested at 30 μg/disc; d: Tested at 10 μg/disc; e Tested at 10 μg/disc.
Leite et al aimed at inhibiting the growth of Gram-positive bacteria causing infective endocarditis, confirmed the inhibitory effects of α-pinene and β-pinene on the growth of S. aureus, S. pneumoniae, S. epidermidis, and S. pyogenes (27). Alavi et al revealed that the volatile oil of P. ruthenicum fruits showed antibacterial activity against S. epidermidis, S. aureus,and B. cereus bacteria. However, it had no antibacterial effect on Gram-negative bacteria, including P. aeruginosa, E. coli,and S. typhi, (28). Moreover, Olga et al focused on the antibacterial activity of P. austriacum EOs. According to their results, the EO possessed moderate antibacterial activity on B. subtilis and S. aureus strains, whereas it had weak antibacterial activity against P. aeruginosa. Similarly, the volatile oil had no antibacterial activity on E. coli and Salmonella typhimurium (20).
Regarding the confirmation of the presence of phenolic compounds by phytochemical analyses, the P. chenur Mozaff was then analyzed by the high-performance liquid chromatography-photodiode array detector, and five phenolic compounds were identified and quantified, including rutin, caffeic acid, naringenin, apigenin, and quercetin (12243, 8855, 2560, 873, and 230 µg/g of the extract, respectively, Table 4). It should be noted that these compounds were first identified and quantified in P. chenur Mozaff (Figures 3 and 4). The phenolic compounds that play an important protective role in plants as secondary metabolites also have a significant effect on human health. For example, rutin has been shown to increase vascular resistance and decrease edema and blood pressure. Additionally, caffeic acid and quercetin have antifungal and antibacterial effects, respectively (27,28). Concerning studies performed on the other species of the genus Peucedanum, the caffeic acid compound was first identified by Bartnik et al in P. tauricumBieb (29). In an earlier study, Kuzmanov et al managed to identify quercetin in P. ruthenicum (30). Subsequently, Alavi et al investigated the phenolic compounds of the aerial parts of P. ruthenicum and identified rutin, quercetin, and caffeic acid (31).
Table 4.
Phenolic Compounds From the Methanolic Extract of Peucedanum chenur Mozaff
|
Phenolic Compounds
|
Concentration (µg/g Extract)
n=3
|
RSD%
|
Absorbance Wavelength (nm)
|
RT
(min)
|
LOD
(µg/L)
|
LOQ
(µg/L)
|
| Quercetin |
230 |
1.2 |
370 |
41 |
20 |
50 |
| Ferulic acid |
nd |
1.1 |
320 |
30 |
5 |
25 |
| Gallic acid |
nd |
1.9 |
270 |
6 |
500 |
1000 |
| Apigenin |
873 |
1.5 |
336 |
52 |
200 |
500 |
| Benzoic acid |
nd |
1.4 |
250 |
28 |
5 |
25 |
| Caffeic acid |
8855 |
1.9 |
300 |
22 |
50 |
100 |
| Rutin |
12243 |
2.2 |
360 |
33 |
20 |
50 |
| Naringenin |
2560 |
1.7 |
290 |
30 |
50 |
200 |
RST: Relative standard deviation; LOD: Limit of detection; LOQ: Limit of quantification; RT: Retention time; nd: Not detected.
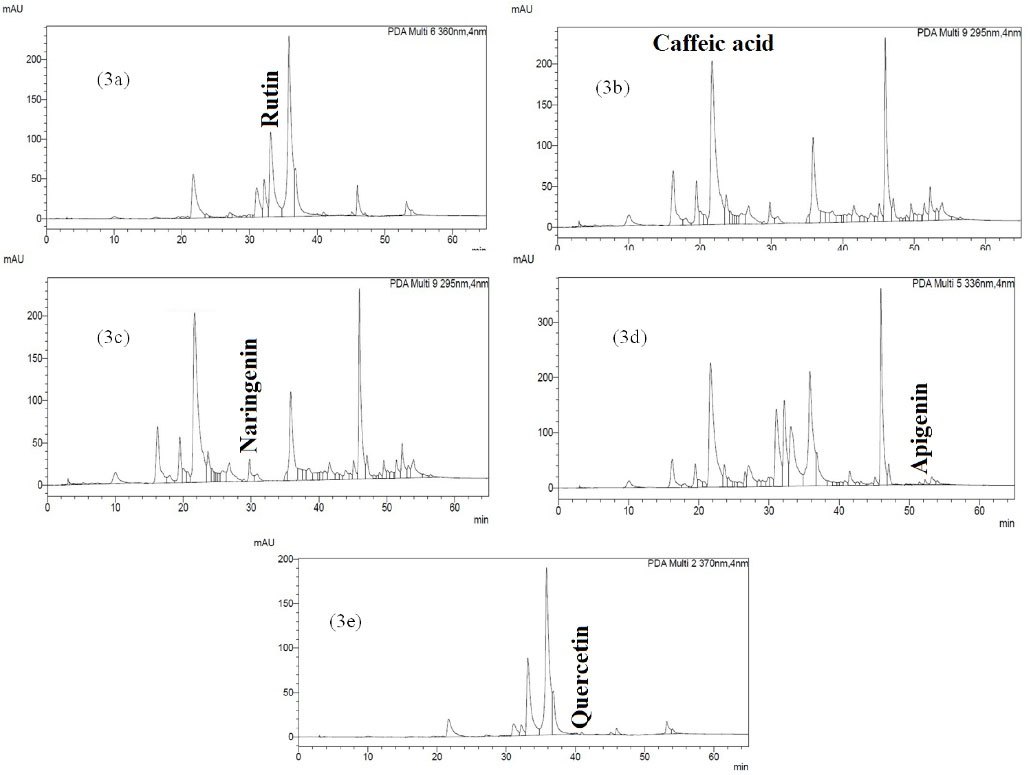
Figure 3.
The HPLC of the Methanolic Extract of Peucedanum chenur Mozaff. 3a: HPLC peak of rutin in methanolic extract of p. chenur at 360 nm. 3b: HPLC peak of caffeic acid in methanolic extract of p. chenur at 295 nm. 3c: HPLC peak of Naringenin in methanolic extract of p. chenur at 295 nm. 3d: HPLC peak of Apigenin in methanolic extract of p. chenur at 336 nm. 3e: HPLC peak of Quercetin in methanolic extract of p. chenur at 370 nm.
Note. HPLC: High-performance liquid chromatography.
.
The HPLC of the Methanolic Extract of Peucedanum chenur Mozaff. 3a: HPLC peak of rutin in methanolic extract of p. chenur at 360 nm. 3b: HPLC peak of caffeic acid in methanolic extract of p. chenur at 295 nm. 3c: HPLC peak of Naringenin in methanolic extract of p. chenur at 295 nm. 3d: HPLC peak of Apigenin in methanolic extract of p. chenur at 336 nm. 3e: HPLC peak of Quercetin in methanolic extract of p. chenur at 370 nm.
Note. HPLC: High-performance liquid chromatography.
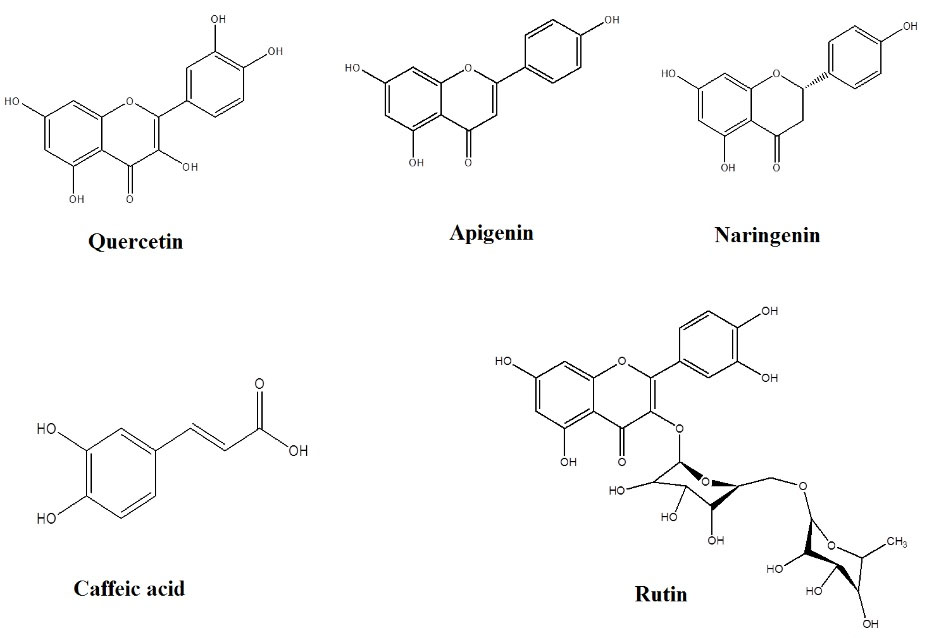
Figure 4.
Structures of the Phenolic Compounds in the Methanolic Extract of Peucedanum chenur Mozaff
.
Structures of the Phenolic Compounds in the Methanolic Extract of Peucedanum chenur Mozaff
Conclusion
This study was performed to first evaluate the chemical constituent and biological effects of the P. chenurMozaff. According to the obtained results, the P. chenur Mozaff volatile oil was a rich source of the α-pinene, limonene, and γ-terpinene compounds. Furthermore, the plant extract contained phenolic compounds such as rutin, caffeic, acid, and naringenin. Due to different applications and biological effects of these compounds reported in previous investigations, further studies should examine the volatile oil and extract constituents. Moreover, the broad range of compounds, as well as the antibacterial and antioxidant activities of P. chenur Mozaff demonstrated the potential of this plant for the application in pharmaceutical and food industries.
Acknowledgements
We are grateful to the Vice-chancellor for Research and Technology, Hamadan University of Medical Sciences (No. 9603231776) for the financial support of this work.
Competing Interests
The authors declare that there is no conflict of interests in this study.
References
- Sarkhail P. Traditional uses, phytochemistry and pharmacological properties of the genus Peucedanum: a review. J Ethnopharmacol 2014; 156:235-70. doi: 10.1016/j.jep.2014.08.034 [Crossref] [ Google Scholar]
- Faridi P, Roozbeh J, Mohagheghzadeh A. Ibn-Sina’s life and contributions to medicinal therapies of kidney calculi. Iran J Kidney Dis 2012; 6(5):339-45. [ Google Scholar]
- Movahedian A, Sajjadi S, Ahmadi M. Lipid lowering effect of ethanolic extract of aerial parts of Peucedanumpastinacifolium Boiss. and Hausskn in hypercholesterolemic rats. Iran J Pharm Res 2022; 8(4):301-6. doi: 10.22037/ijpr.2010.826 [Crossref] [ Google Scholar]
- Momtaz H, Rahimi E, Moshkelani S. Molecular detection of antimicrobial resistance genes in E coli isolated from slaughtered commercial chickens in Iran. Vet Med 2012; 57(4):193-7. [ Google Scholar]
- Wagner H, Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine 2009; 16(2-3):97-110. doi: 10.1016/j.phymed.2008.12.018 [Crossref] [ Google Scholar]
- Miladinović DL, Ilić BS, Kocić BD, Miladinović LC, Marković MS. In vitro interactions of Peucedanum officinale essential oil with antibiotics. Nat Prod Res 2015; 29(10):972-5. doi: 10.1080/14786419.2014.958740 [Crossref] [ Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods--a review. Int J Food Microbiol 2004; 94(3):223-53. doi: 10.1016/j.ijfoodmicro.2004.03.022 [Crossref] [ Google Scholar]
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils--a review. Food Chem Toxicol 2008; 46(2):446-75. doi: 10.1016/j.fct.2007.09.106 [Crossref] [ Google Scholar]
- Duthie G, Crozier A. Plant-derived phenolic antioxidants. Curr Opin Lipidol 2000; 11(1):43-7. doi: 10.1097/00041433-200002000-00007 [Crossref] [ Google Scholar]
- Ryszawa N, Kawczyńska-Drózdz A, Pryjma J, Czesnikiewicz-Guzik M, Adamek-Guzik T, Naruszewicz M. Effects of novel plant antioxidants on platelet superoxide production and aggregation in atherosclerosis. J Physiol Pharmacol 2006; 57(4):611-26. [ Google Scholar]
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2009; 2(5):270-8. doi: 10.4161/oxim.2.5.9498 [Crossref] [ Google Scholar]
- Adams R. Quadrupole mass spectra of compounds listed in order of their retention time on DB-5. In: Identification of essential oils components by gas chromatography/quadrupole mass spectroscopy. Allured Publishing Corporation; 2001.
- Abdali E, Javadi S, Akhgari M, Hosseini S, Dastan D. Chemical composition and biological properties of Saturejaavromanica Maroofi. J Food Sci Technol 2017; 54(3):727-34. doi: 10.1007/s13197-017-2512-0 [Crossref] [ Google Scholar]
- Kerdar T, Rabienejad N, Alikhani Y, Moradkhani S, Dastan D. Clinical, in vitro and phytochemical, studies of Scrophularia striata mouthwash on chronic periodontitis disease. J Ethnopharmacol 2019; 239:111872. doi: 10.1016/j.jep.2019.111872 [Crossref] [ Google Scholar]
- Dastan D, Salehi P, Maroofi H. Chemical composition, antioxidant, and antimicrobial activities on Laserpitiumcarduchorum Hedge & Lamond essential oil and extracts during various growing stages. Chem Biodivers 2016; 13(10):1397-403. doi: 10.1002/cbdv.201600087 [Crossref] [ Google Scholar]
- Ugochukwu SC, Uche A, Ifeanyi O. Preliminary phytochemical screening of different solvent extracts of stem bark and roots of Dennetiatripetala G Baker. Asian J Plant Sci Res 2013; 3(3):10-3. [ Google Scholar]
- Kumar Bargah R. Preliminary test of phytochemical screening of crude ethanolic and aqueous extract of Moringa pterygosperma Gaertn. J Pharmacogn Phytochem 2015; 4(1):7-9. [ Google Scholar]
- Masoudi S, Akhgar MR, Rustaiyan A. Essential oils of Peucedanumscoparium (Boiss) Boiss and Serotinocarpum insignis Mozaffarian from Iran. J Essent Oil Res 2004; 16(2):117-9. doi: 10.1080/10412905.2004.9698667 [Crossref] [ Google Scholar]
- Rustaiyan A, Komeilizadeh H, Mojab F, Khazaie A, Masoudi S, Yah M. Essential oil composition of Peucedanumpetiolare (DC) Boiss from Iran. J Essent Oil Res 2001; 13(1):49-50. doi: 10.1080/10412905.2001.9699603 [Crossref] [ Google Scholar]
- Jovanović OP, Zlatković BK, Simonović SR, Đorđević AS, Palić IR, Stojanović GS. Chemical composition and antibacterial activity of the essential oils isolated from leaves and fruits of Peucedanumaustriacum (Jacq) WDJ Koch. J Essent Oil Res 2013; 25(2):129-37. doi: 10.1080/10412905.2012.751558 [Crossref] [ Google Scholar]
- Figuérédo G, Chalchat JC, Petrovic S, Maksimovic Z, Gorunovic M, Boza P. Composition of essential oils of flowers, leaves, stems and rhizome of Peucedanum officinale L (Apiaceae). J Essent Oil Res 2009; 21(2):123-6. doi: 10.1080/10412905.2009.9700128 [Crossref] [ Google Scholar]
- Crowell PL, Elson CE, Bailey HH, Elegbede A, Haag JD, Gould MN. Human metabolism of the experimental cancer therapeutic agent d-limonene. Cancer Chemother Pharmacol 1994; 35(1):31-7. doi: 10.1007/bf00686281 [Crossref] [ Google Scholar]
- de Oliveira Ramalho TR, de Oliveira MTP, de Araujo Lima AL, Bezerra-Santos CR, Piuvezam MR. Gamma-terpinene modulates acute inflammatory response in mice. Planta Med 2015; 81(14):1248-54. doi: 10.1055/s-0035-1546169 [Crossref] [ Google Scholar]
- Baloch NI, Kakar AM, Nabi SA, Yasinzai MA, Al-Kahraman DY. In vitro antimicrobial, insecticidal, cytotoxic activities and their phytochemical analysis of methanolic extract and its fractions of Peucedanumbeluchistanicum leaves. Int J Pharma Bio Sci 2013; 4(2):898-905. [ Google Scholar]
- Tepe B, Akpulat HA, Sokmen M. Evaluation of the chemical composition and antioxidant activity of the essential oils of Peucedanumlongifolium (Waldst & Kit) and P palimbioides (Boiss). Rec Nat Prod 2011; 5(2):108-16. [ Google Scholar]
- Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR. Colistin: the re-emerging antibiotic for multidrug-resistant gram-negative bacterial infections. Lancet Infect Dis 2006; 6(9):589-601. doi: 10.1016/s1473-3099(06)70580-1 [Crossref] [ Google Scholar]
- Leite AM, de Oliveira Lima E, de Souza EL, de Fátima Formiga Melo Diniz M, Trajano VN, de Medeiros IA. Inhibitory effect of beta-pinene, alpha-pinene and eugenol on the growth of potential infectious endocarditis causing gram-positive bacteria. Rev Bras Ciênc Farm 2007; 43(1):121-6. doi: 10.1590/s1516-93322007000100015 [Crossref] [ Google Scholar]
- Alavi SH, Yassa N, Fazeli MR. Chemical constituents and antibacterial activity of essential oil of Peucedanumruthenicum M Bieb fruits. Iran J Pharm Sci 2005; 1(4):217-22. [ Google Scholar]
- Bartnik M, Głowniak K, Dul R. Use of two-dimensional TLC to identify phenolic acids in the foliage and fruit of Peucedanumtauricum Bieb. JPC-Journal of Planar Chromatography-Modern TLC 2003; 16(3):206-10. doi: 10.1556/jpc.16.2003.3.7 [Crossref] [ Google Scholar]
- Kuzmanov B, Andreev N, Kozovska V. Chemotaxonomic study on Bulgarian species of” Peucedanum” L I. An Jard Bot Madr 1980; 37(2):779-88. [ Google Scholar]
- Alavi SH, Yassa N, Hajiaghaee R, Matin Yekta M, Rezaei Ashtiani N, Ajani Y. Phenolic compounds from Peucedanumruthenicum M Bieb. Iran J Pharm Res 2009; 8(1):71-5. [ Google Scholar]