Avicenna Journal of Pharmaceutical Research. :45-54.
doi: 10.34172/ajpr.2022.1063
Review Article
Toxic Environmental Factors and Multiple Sclerosis: A Mechanistic View
Sayyed-Ali Tabatabaei 1  , Saba Ariafar 1, Mojdeh Mohammadi 1, *
, Saba Ariafar 1, Mojdeh Mohammadi 1, * 
Author information:
1Department of Pharmacology & Toxicology, School of Pharmacy, Hamadan University of Medical Sciences, Hamadan, Iran
Abstract
Multiple sclerosis (MS) is an acquired inflammatory and neurodegenerative immuno-mediated disorder of the central nervous system (CNS) characterized by inflammation, demyelination, and primary or secondary axonal degeneration. Epidemiological studies have suggested that both genetic and non-genetic risk factors are involved in the etiology of MS. Non-genetic factors include infections, vaccinations, nutritional habits, hormonal factors, and physical and chemical agents. Toxic environmental factors have been proposed to play a considerable role in MS pathogenesis. This review explored pieces of evidence and potential mechanisms of action for some toxic factors such as heavy metals, organic solvents (OSs), tobacco smoking, plastic monomers, additives, and pesticides. The obtained findings provide us with the potential for prevention, especially for people at greater risk such as individuals exposed to these toxic factors. It should be noted that further investigations are needed to find precise mechanisms of causality in humans and to develop defensive approaches.
Keywords: Multiple sclerosis, Heavy metals, Neurotoxicity, Organic solvents, Tobacco
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Tabatabaei SA, Ariafar S, Mohammadi M. Toxic environmental factors and multiple sclerosis: a mechanistic view. Avicenna J Pharm Res. 2022; 3(1):45-54. doi:10.34172/ajpr.2022.1063
Introduction
Multiple sclerosis (MS) is a chronic demyelinating disease of the white matter of the central nervous system (CNS) affecting about 2.5 million people worldwide. This incapacitating disease of the CNS usually follows a waxing and waning course over many years before advanced disability supervenes (1). It is hypothesized that the loss of axons is the main mechanism underlying progressive disability (2). Most of the damage in MS is due to peroxynitrite, which results from excess superoxide anions (3). Three forms of MS are relapsing-remitting, relapsing progressive, and primary progressive. MS is more frequent in women than in men with a 3:1 ratio. It clinically manifests itself with signs of multiple neurological dysfunctions (e.g., visual and sensory disturbances, bilateral Babinski signs, limb weakness, gait problems, and bladder and bowel symptoms) followed by recovery or growing disability because of irreversible functional disability over time (1,4). It is assumed that both environmental and genetic factors exert an effect on MS.
Based on previous findings, various environmental factors play a role in the development of this disease. Environmental agents are believed to trigger a T-cell-mediated chronic inflammatory response to myelin proteins in individuals with a genetic predisposition, creating the characteristic lesions that cause disease (5,6). Therefore, examining environmental stressors and genes that modulate the immune system concurrently represents the best starting point for exploring the etiology of MS. Numerous environmental exposures such as heavy metals, organic solvents (OSs), ultraviolet radiation, infections, and diet have been investigated as possible etiologic factors for MS with inconsistent findings (4,7,8). Some studies support the etiologic role of metals in autoimmune disease and highlight the importance of interactions between genes and the environment. In genetically susceptible animals, mercury exposure even at low doses accelerates autoimmune disease and leads to disruption in cytokine production (9). In addition, low to moderate levels of lead exposure can cause functional alterations in T lymphocytes and macrophages, leading to increased hypersensitivity, altered cytokine production, and increased risk of inflammation-associated tissue damage (10). In no-predisposed animals, pretreatment with inorganic mercury can exacerbate autoimmune disease even prior to disease induction (11). Moreover, exposure to OSs such as toluene or xylene has been postulated to affect MS risk through altered immune function or neurotoxicity (12,13).
Although it is believed that MS is multifactorial in etiology, finite studies have examined the role of environmental toxic factors such as heavy metals, solvents, plastic monomers, additives, and pesticides in the susceptibility of genes associated with immune response and subsequent development of MS. This review aimed to investigate the associations between MS and some environmental toxic factors such as heavy metals, OSs, plastic monomers, additives, and pesticides.
Heavy Metals
Mercury
Dental amalgam, a tooth-filling (14) compound which contains about 50% mercury in a multipart mixture of copper, tin, silver, and zinc, is the main source of human mercury body burden (15-17). On the other hand, mercury is one of the most toxic elements among others, especially when it is in the gas phase (16). Amalgam emits mercury vapor, increased by chewing, eating, brushing, and drinking hot liquids. Fishes (mostly predators), antiparasitics, antiseptics, preservatives, pesticides, fungicides, and beauty creams are mercury’s environmental exposure sources (18,19). It should be noted that mercury accumulates in the body during the long exposure time, and its deposits in the brain and bone tissues last from several years to decades (16). Mercury affects biological systems by the following mechanisms:
-
Breaking hydrogen bands
-
Displacing with other metallic ions
-
Disturbing the catalytic activity because of structural changes in proteins
-
Alteration of translation, leading to mutagenic and carcinogenic activity
-
Binding strongly with sulfhydryl and selenohydryl groups (20,21).
It was observed that mercury serum level is higher in MS patients compared to the control individuals which indicates that mercury serum level is a factor that can increase susceptibility (18). Moreover, the mercury content of the cerebrospinal fluid of MS patients is higher than that in normal people (22). It has been suggested that there is a correlation between MS clusters and dental silver-mercury fillings (23). On the other hand, there are also studies suggesting the association between MS and dental amalgam (24). Based on the findings from an investigation, MS patients with amalgam fillings had meaningfully more neuromuscular exacerbations compared to those who had removed amalgam fillings. Furthermore, it has been revealed that electrophoretic bands of cerebrospinal fluid proteins were normalized after removing the dental amalgam (15). Another study discovered that amalgam removal can improve autoimmune diseases such as MS (15,25). In a study, it was reported that MS patients with dental amalgam fillings have meaningfully lower levels of red blood cells (RBCs), hemoglobin, hematocrit, serum IgG, and higher blood urea nitrogen compared to MS patients with amalgam removal (19). It was also identified that mercury can destroy the myelin sheath (19,26), decrease the velocity of nerve conduction, induce autoimmune responses, and damage the blood-brain barrier (BBB) which is related to MS (19). Mercury can also produce antibodies against myelin basic protein (MBP) (19,27,28). Moreover, based on Issa and colleagues’ study, mercury has toxic effects on oligodendroglia cells, which are affected in MS disease (28). In addition, mercury contributes to the cytoskeleton structure development destruction process in nerve cells (18,29). Neurons, especially sensitive ones, can be damaged by methylmercury (22,30).
On the other hand, a case-control study performed on MS patients failed to disclose a relationship between either the number of dental amalgam fillings or the duration of exposure to mercury amalgam and MS (24,31).
Based on the toxicity mechanisms mentioned for mercury, its bind to the selenohydryl groups, especially the one in glutathione which is an efficient antioxidant in the body, can be considered a probable role of mercury in MS pathogenesis through oxidative stress.
Cadmium
Cadmium (Cd), one of the most important toxic metals which is a byproduct of the mining and smelting of lead and zinc, is used in PVC plastics, cigarettes, nickel-Cd batteries, paint pigments, and pesticides (32). Some exposure ways of Cd include the inhalation of polluted air, smoking cigarette, and contaminated food (especially seafood) and water due to the use of insecticides, fungicides, and chemical fertilizers (32-35). It is also reported that the wastewater used for irrigation generally has a higher Cd concentration compared to the well water (36). Inhalation accounts for 15% to 50% of absorption through the respiratory system. About 2% to 7% of the ingested Cd is absorbed in the gastrointestinal system. The target organs of Cd are the liver, testis, placenta, kidneys, lungs, brain, and bones (32,34). Moreover, Cd has a significantly longer half-life in biological organs (21).
Cd can decrease glutathione levels which induce neurotoxicity and destroy retrograde axonal transport (37). Therefore, Cd exposure can lead to reactive oxygen species (ROS) production which in turn can induce lipid peroxidation and change intracellular calcium level and membrane fluidity (38-41).
Several studies have been conducted on the relationship between Cd and MS. In a study in 2016 in Iran, it was observed that MS patients have higher Cd blood concentration level (1.82 ± 0.13 μg/L) compared to healthy individuals (1.47 ± 0.11 μg/L). It has been suggested that Cd induces neurological abnormalities, cerebral hemorrhage, and neonatal cerebral edema. Moreover, Cd can induce the production of reactive radicals, depletion of reduced glutathione, binding to thiol groups of various proteins, and disruption of antioxidant enzyme activity (35). In another study in 2017, the correlation between heavy metal exposure and glutathione S-transferase M1 (GSTM1) polymorphism in Iranian MS patients was studied and it was revealed that the GSTM1 null genotype is associated with high Cd level in relapsing remitting MS (RRMS) patients (42). In a case study in 2012, the removal of heavy metals improved MS disease (43). Cd and mercury were reported to disrupt the mitochondria of neurons by increasing intracellular ROS which directly inhibits Janus kinase activity in neurons (44). The ROS along with advanced glycation end products has been implied as triggers for the production of pro-inflammatory cytokines (43). Moreover, it was reported that the risk of MS in smokers is partly due to Cd (32,35).
In addition, in animal fields, a significant increase in lipid peroxidation markers (thiobarbituric acid reactive substances and lipid hydroperoxides) and a significant decrease in enzymatic and non-enzymatic antioxidants (i.e., superoxide dismutase, catalase, glutathione peroxidase, GST, reduced glutathione, vitamins C and E, and sulfhydryl groups) and glutathione metabolizing enzymes (i.e., glutathione reductase and glutathione-6-phosphate dehydrogenase) were reported in rats treated with Cd (40).
Arsenic
Arsenic is one of the most toxic naturally occurring metals, and its highest toxicity level mainly affects the human nervous system (45). Arsenic exposure ways include contaminated drinking water (the most common long-term way of exposure) as well as air and food such as seafood, rice, and mushrooms (46-48).Arsenic has two oxidation forms: a trivalent form named arsenite and a pentavalent form named arsenate, and the pentavalent toxicity level is 60 times higher than trivalent toxicity (45).
Based on the findings of a study in Iran, there was a correlation between arsenic contamination of drinking water and MS prevalence because of ROS elevation by arsenic (46). Moreover, it has been reported that MS patients have higher levels of arsenic and malondialdehyde, a lipid peroxidation marker, in serum. Indeed, arsenic can induce inflammation, tau protein hyperphosphorylation, ROS-mediated oxidative damage, and mitochondrial damage which significantly contribute to the injury and loss of both axons and neurons (45). By contrast, one study in Taiwan revealed that arsenic concentration in soil is negatively related to the incidence of MS (49). Based on previous findings, oxidative stress apparently plays an important role in MS pathogenesis or progression. CNS is more vulnerable to the damage caused by ROS due to the high rate of oxygen consumption, high levels of phospholipids and mitochondria, and low levels of antioxidants, especially the limited concentration of reduced glutathione (48,50). Arsenic induces ROS generation in mitochondria by deviating electrons from the respiratory chain involved in the pathogenesis or progression of MS (45,51).
Furthermore, arsenic exposure can cause oxidative damage in oligodendrocytes and axons, which leads to apoptosis, myelin destruction, and CNS degeneration (46,52,53). Moreover, exposure to arsenic can cause neuro-inflammation by activating and translocating the NF-κB to the cell nucleus where it induces the transcription of cytokines, chemokines, and adhesion molecules (54).
Lead
Lead is a generally distributed metal and a persistent poison found in various forms such as lead salts and metallic lead in minerals. It has been expended in numerous industries, including toys, hygiene products, the printing industry, households, and cosmetics (55). Although lead naturally exists in almost all elements on earth (56), the most frequent routes of lead exposure are soil, products containing lead (e.g., batteries, cable coverings, fuel additives, paints, insecticides, cosmetics, and plastics), and water contamination caused by lead pipes (57-59). The lead pollution rate has grown rapidly due to the significant consumption of lead-contained gasoline, especially in industrial areas (60). In addition, there is no safe level for lead exposure due to its high toxicity (61). About 90% of the absorbed lead is bound to the RBCs, which is in dynamic equilibrium with plasma lead (62). It should be noted that blood lead level in men is higher than in women due to the higher amount of RBC, higher exposure, and gender-related metabolism differences (35). The half-life of lead in the human body can sometimes approach 27 years in bones (63). Some organs such as bones, brain, blood, kidneys, and thyroid are known as the destination for storage and toxicity of lead (32). The lead absorption rate through the gastrointestinal system is reported to be four times higher than in adults (57).
Lead can move across the endothelial cells at BBB. It can be absorbed by calcium-ATPase pumps and act as a substitution for calcium ions so it can interfere with DNA transcription and enzymes that contribute to vitamin D activation and enzymes that keep the integrity of the cell membrane (64).
Vitamin D (an antioxidant) is assumed to be a protective element in MS pathogenicity through the maintenance of oxidative stress and DNA repair genes (65). In addition, it is suggested as a prognosticator for MS (66). There are both clinical and in vivo studies indicating the vitamin D level alteration due to lead exposure. In a case-control study conducted on jewelry workers in Bangladesh, a decrease was reported in vitamin D levels in lead-exposed workers. The nephrotoxicity and renal 1-α-hydroxylase inhibition resulting from lead were suggested to be responsible for the low vitamin D level (67). Additionally, a study was conducted to evaluate the vitamin D level and its metabolism in rats. At some developmental stages of the studied animals, the findings indicated a significant decrease in 25-hydroxy vitamin D (25(OH)D) and an increase in hepatic 25-hydroxylase due to the lead toxicity, as a response to 25(OH)D reduction. It is stated that the increase in hepatic 25-hydroxylase does not necessarily demonstrate the proper or high performance of the enzyme. In addition, a significant decrease in 1,25-dihydroxy vitamin D was reported. On the other hand, vitamin D receptors in the brain significantly increased in lead-exposed rats at all developmental stages, which is thought to be a reflex to the decreased level of circulating 25(OH)D in order to increase the cellular sensitivity to vitamin D (68).
In some regions, an abnormally high MS incidence has been proposed to be related to high levels of lead in soil, groundwater, vegetables, and rocks (69,70). It has been reported that toxic metals such as lead can produce free radicals, cause BBB damage, change DNA and protein structure, and induce lipid peroxidation (35,57). The accumulation of some heavy metals (e.g., lead) inside the brain cells can induce the production of autoantibodies against MBPs, neurofilaments, and the cytoskeleton of the neurons (4). In a study, the accumulation of some toxic elements such as lead may play an important role in the MS etiology of some cases. It has been realized that lead and Cd toxicity can result in some serious diseases such as cardiovascular diseases, diabetes, neurological disorders, cancer, and cognitive impairments (60). These metals have been shown to be related to the increase of ROS and decrease of antioxidant defensive capacity (71). Lead acts as a redox-active metal (unlike redox-inert metals such as Cd mostly depleting the reduced glutathione and binding to the thiol group of various proteins) and creates free radical species by taking part in the electron transfer chain. The molecular mechanism of lead toxicity is multifactorial as it generates free radical species, reduces glutathione antioxidants, prevents enzyme activity, and blocks central trace element absorption (57,72,73). Lead has been recognized as an important element in oxidative stress. In a study, oxidative damage was suggested as the reason for the demyelination plaques happening in MS (35,57,74). It has been reported that lead may induce the immunologic response by increasing the immunogenicity of neuronal proteins (75). Since lead can cause the formation of antibodies against MBPs, it is probable to play a role in the pathogenesis of some neurological diseases such as MS (76). In a study in 2015, it was stated that exposure to a substantial amount of lead can raise the risk of developing MS (77). During the development of the nervous system, a short-time exposure to a large amount of lead may interfere with the myelin synthesis and make a series of irreversible changes leading to the conditions of MS (77) According to the results of a study, the mean blood lead levels were significantly higher in MS patients than in the controls. Furthermore, it was reported that the risk of MS increased by 1.17 times per each μg/L elevation of lead in the blood (55). On the other hand, various studies on blood lead levels have not detected significant differences between MS patients and control groups (35,77,78).
Organic Solvents
OSs are used in broad-spectrum operations in industrial societies, including dry cleaning, cosmetics, paintings, disinfectants, perfumes, and fresheners. Acetone (the simplest ketone), tetrachloroethylene, and toluene are some compounds classified under the name of OSs (79-81). Inhalation and dermal contact are the main routes of both occupational and environmental exposures. The solvents can be excreted via various pathways after biotransformation, including unchanged exhalation or accumulation in lipid-rich tissues such as adipose and brain due to their high lipophilicity and ability to cross BBB (79). OSs are metabolized through GST mainly in the liver (82). Both peripheral and CNSs can be forced to change by OSs. Neurotoxic effects of OSs include focus difficulties, tiredness, and memory dysfunction (83-85). Moreover, OS exposure is a risk factor in developing an autoimmune disease such as MS (13,86,87). They may interfere with fatty acid processing in the myelin and lipid membrane (88,89). It has been found that exposure to OSs, particularly exposure to kerosene in Italian shoe and leather workers, raised the risk of MS (90). OSs, which are generally used in the adhesion industry, may be involved in pathogenic mechanisms by interfering with the immune system (91). Results of a meta-analysis in 2012 showed a meaningful relationship between OS exposure and the development of autoimmune disease. Modification of self-proteins by OSs may lead to immunologic response and tissue injury (13). As mentioned before, OSs are metabolized by GSTM1(an important enzyme in reactive intermediates detoxification) and cytochrome P450 2D6. It should be noted that the null genotype (poor metabolizer) of GSTM1 is associated with chronic toxic encephalopathy induced by OSs (92). The immune system can be altered by OSs via impairing phagocytosis, diminishing serum complement level, and altering BBB structure, leading to the appearance of lesions in the white matter of the brain. A case report in 2001 emphasized the effect of OSs on triggering MS by impairing the impermeability of the BBB (93). Volatile anesthetics are structurally linked to OSs. In a study in 2003, a high risk of MS was detected in nurse anesthetists compared to others. The important mechanism of MS induction by volatile anesthetics such as halothane is the impairment of the hepatic antioxidant defense system, resulting in oxidative stress as a pathogenic mechanism for MS (89).
Tobacco Smoking
Tobacco smoke contains various chemicals such as nicotine, nitrogen oxides, hydrogen cyanide, and carbon monoxide. Many of these chemicals have established risks to the immune system (94). Tobacco smoke changes antigen-mediated T-cell signaling (95). This effect may be mediated by T helper 17-cells which are linked by aryl hydrocarbon receptors to toxic chemicals in tobacco smoke (96-98). Tobacco smoke stimulates the activation of neutrophils, macrophages, and monocytes (99). Based on some findings, several inflammatory markers such as interleukin 6 and C-reactive protein can elevate in tobacco smokers (100,101). Other manifestations of the immune system in tobacco smokers include abnormal T-cell function, natural killer cell loss, and impairment of humoral and cell-mediated immunity (102-104). In addition to the toxic effect on the immune system, some tobacco smoke chemicals have a toxic effect on the nervous system. It was demonstrated that cyanide and its principal metabolite thiocyanate can cause demyelination in the CNS (105). As mentioned, tobacco smoke contains various free radical derivatives of nitric oxide (106). It has been suggested that nitric oxide has a role in secondary progressive MS because of axonal degeneration and axonal conduction blocking (107). Carbon monoxide, another component of the vapor phase, induces cerebral demyelination (108). It has been indicated that nicotine affects the integrity and function of the BBB which leads to the leakage of inflammatory mediators and the development of MS (109). Although the association between MS and smoking has not been identified clearly, several studies have revealed the elevated risk of disability and accelerated conversion time from RRMS to secondary progressive MS in tobacco smokers with MS (110). In addition, numerous studies have established the connotation between tobacco smoking and mortality rate in patients with MS (111-113). On the other hand, MS progression from clinically isolated syndrome to clinically definite MS was not significant in tobacco smokers with MS compared to nonsmokers (114).
Plastic Monomers and Additives
Industrial-scale production of plastic materials flourished in the 1940s and 1950s. Plastics provide a broad spectrum of benefits for human health which leads to diverse consumption and consequently a large volume of waste. Several implications are associated with plastics, including waste recycling methods and the release of additives embedded in them under various conditions. Additives are chemical compounds added to improve the functional properties of the basic polymer. The undesirable migration of plastic additives has been studied, especially on plasticizers and antioxidants (115). The type and quantity of released additives may be influenced by the presence of polymerization contaminations, degradation mechanisms, and surrounding factors such as oxygen and temperature. Thermal degradation of nitrogen-containing, chlorine-containing, and fluorine-containing plastics causes the release of hydrogen cyanide, dioxins, and hydrogen fluoride, respectively (116). Plastic monomers, units of macromolecules forming plastics, tend to migrate from food packaging materials into foods. Several studies have shown that health hazards can arise if unreacted monomer concentrations reach a certain limit. It has been reported that the migration of styrene and bisphenol A can lead to neurological disorders (117,118). Mast cells are in close contact with MS plaques (119), and it has been observed that mast cell tryptase is elevated in the cerebrospinal fluid of MS patients which can dissolve the myelin sheath protein. In the common type of MS (i.e., RRMS), the relapsing phase can be explained by the release of histamine and proteases after a massive stimulation of the brain mast cells, and remitting phase can be explained by reloading the stores of histamine and proteases. Some findings indicated that phthalates potentiate the antibody-induced release from mast cells (120).
In addition, plastic monomers and additives may contain some Cd- and lead-based compounds participating in MS pathogenesis as explained before.
Pesticides
Pesticides contain herbicides (e.g., 2,4-dichlorophenoxyacetic acid, diquat, and paraquat), insecticides (e.g., organochlorines, organophosphates [Ops], pyrethroids, and carbamates), fumigants (e.g., methyl bromide and ethylene dibromide), rodenticides (e.g., anticoagulants), and fungicides (e.g., captan and dithiocarbamates). Pesticides are used vastly causing severe environmental pollution and health hazards (121), and they stimulate the disturbance of the total antioxidant capability, production of free radicals, and induction of lipid peroxidation (121-123). OPs, insecticides containing phosphorous, are cholinesterase inhibitors, which are also used as biological warfare agents affecting the nervous system. OPs and carbamates inhibit the function of acetylcholinesterase, butyrylcholinesterase, paraoxonases (esterases), and other kinds of nonspecific esterases (121). The organochlorines, namely, chlorinated hydrocarbon insecticides, are divided into four distinct groups: dichlorodiphenyltrichloroethane, cyclodienes (e.g., aldrin, toxaphene, endrin, heptachlor, dieldrin, endosulfan, chlordane, and chlordecone), hexachlorocyclohexane (e.g., lindane), and other related compounds (121). Organochlorines are extremely lipid soluble and stable against degradation, and they have a long half-life (124). It has been reported that organochlorines can stimulate the CNS (121) with various mechanisms in their different subtypes.
It was reported that both acute and chronic exposure to pesticides can occur during farming activities such as the mixing and spraying of pesticides. The exposure pattern is quite extensive due to the unawareness of the toxic effects of pesticides and no use of suitable protective equipment during the pesticide application (125). Numerous epidemiological education has indicated that pesticide exposure can be a significant risk factor for neurological disorders, including Alzheimer’s disease, Parkinson’s disease, and MS (126,127). In a study in 2014, the excess risk of MS was observed in female agricultural workers which could indicate the role of pesticides (128). Along with this study, it was found that the occurrence of MS is significantly high in districts with more pesticide use compared to those with lesser pesticide use (129). Moreover, it was reported that having parents exposed to pesticides (agricultural-related occupations) is related to a higher risk of pediatric MS (130).
Paraoxonase 1, which can be inhibited by OPs and carbamates, may increase the peroxidase activities, which interferes with the MS pathogenicity and oxidative stress (131). In an animal-based study, it was reported that the brain’s glutathione disulfide amount significantly increased in rats that were exposed to dichlorvos (132).
Regarding the organochlorines, a study reported that dichlorodiphenyltrichloroethane and methoxychlor exposure result in the decrease of antioxidant enzymes in rats (133), the induction of oxidative stress, and lipid peroxidation (134,135). In another study, it was stated that toxaphene can disturb the glutathione-redox balance (136). In addition, some other cyclodienes such as endrin, endosulfan, and dieldrin have been reported to cause oxidative stress (137-139), which is a possible mechanism of MS pathogenesis (48). Figure 1 represents the environmental factors and their toxicity pathways in MS pathogenesis.
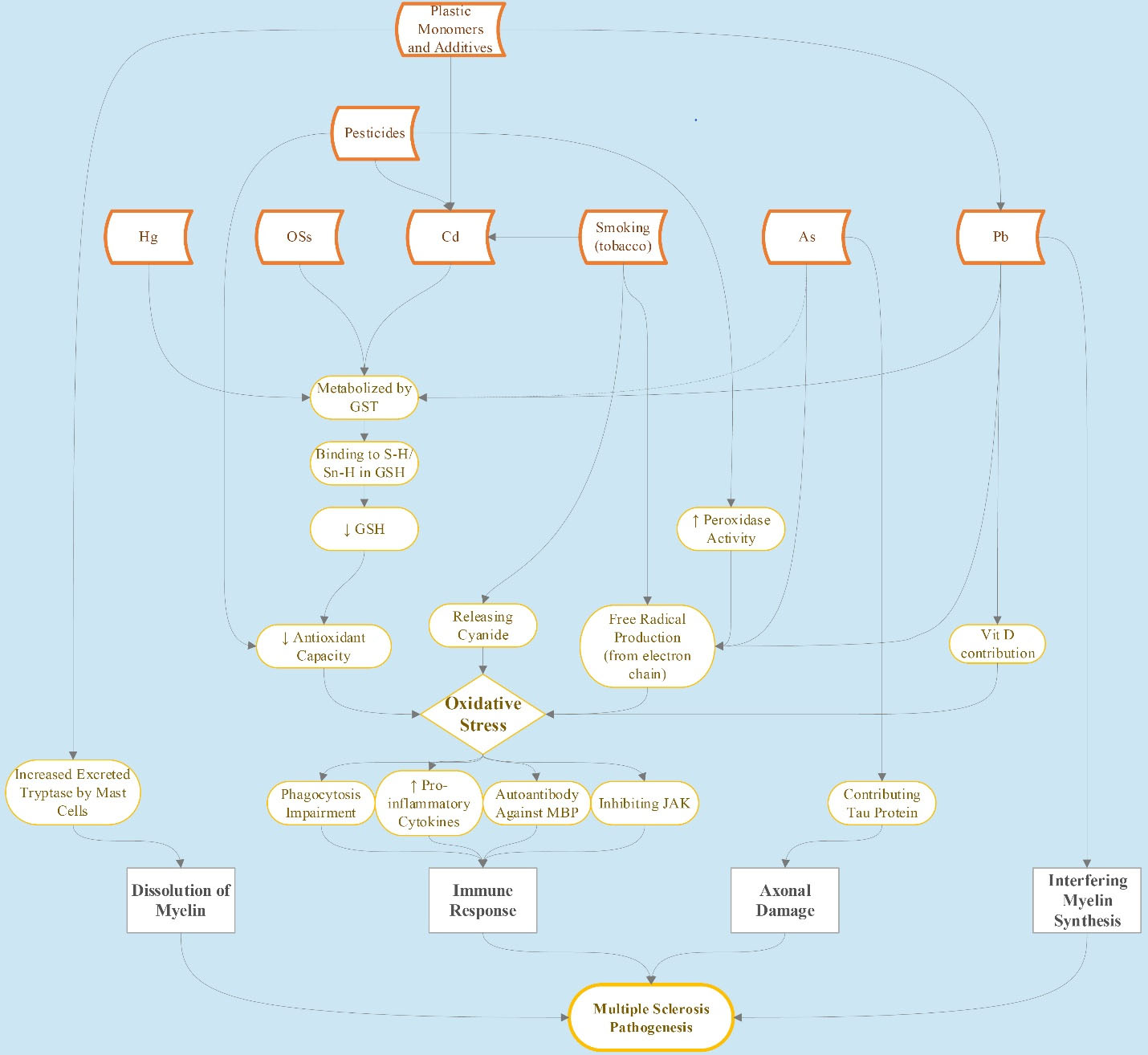
Figure 1.
Environmental Factors and Toxicity Pathways in MS Pathogenesis. Note. MS: Multiple sclerosis
.
Environmental Factors and Toxicity Pathways in MS Pathogenesis. Note. MS: Multiple sclerosis
Conclusion
Environmental toxic factors affecting MS are progressively described. Most of these factors such as exposure to mercury, Cd, lead, arsenic, pesticides, and OSs as well as smoking may increase oxidative stress, whether through producing free radicals or decreasing the antioxidant capacity. The oxidative stress itself can increase inflammatory cytokines, impair phagocytosis, damage BBB, alter the creation of autoantibodies against myelin, and inhibit Janus kinase in neurons, leading to immune responses targeting neurons of the CNS in MS pathogenesis. In addition, the myelin of the neurons can be dissolved by tryptase excreted by mast cells altered through exposure to plastic monomers and additives. Factors that trigger MS disease can increasingly be incorporated into practical health care and even prevention, specifically for individuals at increased risk for MS. However, more studies are needed to clarify the role and importance of these factors in MS pathogenicity.
Competing Interests
The authors report no conflict of interests.
Funding
There is no financial supporting.
References
- O’Gorman C, Lucas R, Taylor B. Environmental risk factors for multiple sclerosis: a review with a focus on molecular mechanisms. Int J Mol Sci 2012; 13(9):11718-52. doi: 10.3390/ijms130911718 [Crossref] [ Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372(9648):1502-17. doi: 10.1016/s0140-6736(08)61620-7 [Crossref] [ Google Scholar]
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69(2):292-302. doi: 10.1002/ana.22366 [Crossref] [ Google Scholar]
- Napier MD, Poole C, Satten GA, Ashley-Koch A, Marrie RA, Williamson DM. Heavy metals, organic solvents, and multiple sclerosis: an exploratory look at gene-environment interactions. Arch Environ Occup Health 2016; 71(1):26-34. doi: 10.1080/19338244.2014.937381 [Crossref] [ Google Scholar]
- Marrie RA. Environmental risk factors in multiple sclerosis aetiology. Lancet Neurol 2004; 3(12):709-18. doi: 10.1016/s1474-4422(04)00933-0 [Crossref] [ Google Scholar]
- Weiner HL. A 21 point unifying hypothesis on the etiology and treatment of multiple sclerosis. Can J Neurol Sci 1998; 25(2):93-101. doi: 10.1017/s0317167100033680 [Crossref] [ Google Scholar]
- Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis Part II: noninfectious factors. Ann Neurol 2007; 61(6):504-13. doi: 10.1002/ana.21141 [Crossref] [ Google Scholar]
- Landtblom AM, Flodin U, Söderfeldt B, Wolfson C, Axelson O. Organic solvents and multiple sclerosis: a synthesis of the current evidence. Epidemiology 1996; 7(4):429-33. doi: 10.1097/00001648-199607000-00015 [Crossref] [ Google Scholar]
- Hansson M, Djerbi M, Rabbani H, Mellstedt H, Gharibdoost F, Hassan M. Exposure to mercuric chloride during the induction phase and after the onset of collagen-induced arthritis enhances immune/autoimmune responses and exacerbates the disease in DBA/1 mice. Immunology 2005; 114(3):428-37. doi: 10.1111/j.1365-2567.2005.02105.x [Crossref] [ Google Scholar]
- Dietert RR, Piepenbrink MS. Lead and immune function. Crit Rev Toxicol 2006; 36(4):359-85. doi: 10.1080/10408440500534297 [Crossref] [ Google Scholar]
- Via CS, Nguyen P, Niculescu F, Papadimitriou J, Hoover D, Silbergeld EK. Low-dose exposure to inorganic mercury accelerates disease and mortality in acquired murine lupus. Environ Health Perspect 2003; 111(10):1273-7. doi: 10.1289/ehp.6064 [Crossref] [ Google Scholar]
- Leira HL, Fonnum F, Syversen T. Organic solvents. In: Ballantyne B, Marrs TC, Syversen T, Casciano DA, Sahu SC. General, Applied and Systems Toxicology. Wiley; 2009.
- Barragán-Martínez C, Speck-Hernández CA, Montoya-Ortiz G, Mantilla RD, Anaya JM, Rojas-Villarraga A. Organic solvents as risk factor for autoimmune diseases: a systematic review and meta-analysis. PLoS One 2012; 7(12):e51506. doi: 10.1371/journal.pone.0051506 [Crossref] [ Google Scholar]
- Jirau-Colón H, González-Parrilla L, Martinez-Jiménez J, Adam W, Jiménez-Velez B. Rethinking the dental amalgam dilemma: an integrated toxicological approach. Int J Environ Res Public Health 2019; 16(6):1036. doi: 10.3390/ijerph16061036 [Crossref] [ Google Scholar]
- Mutter J, Naumann J, Sadaghiani C, Walach H, Drasch G. Amalgam studies: disregarding basic principles of mercury toxicity. Int J Hyg Environ Health 2004; 207(4):391-7. doi: 10.1078/1438-4639-00305 [Crossref] [ Google Scholar]
- Mutter J, Naumann J, Guethlin C. Comments on the article “the toxicology of mercury and its chemical compounds” by Clarkson and Magos (2006). Crit Rev Toxicol 2007; 37(6):537-49; discussion 51. doi: 10.1080/10408440701385770 [Crossref] [ Google Scholar]
- Aminzadeh KK, Etminan M. Dental amalgam and multiple sclerosis: a systematic review and meta-analysis. J Public Health Dent 2007; 67(1):64-6. doi: 10.1111/j.1752-7325.2007.00011.x [Crossref] [ Google Scholar]
- Movahedian Attar A, Kharkhaneh A, Etemadifar M, Keyhanian K, Davoudi V, Saadatnia M. Serum mercury level and multiple sclerosis. Biol Trace Elem Res 2012; 146(2):150-3. doi: 10.1007/s12011-011-9239-y [Crossref] [ Google Scholar]
- Siblerud RL, Kienholz E. Evidence that mercury from silver dental fillings may be an etiological factor in multiple sclerosis. Sci Total Environ 1994; 142(3):191-205. doi: 10.1016/0048-9697(94)90327-1 [Crossref] [ Google Scholar]
- Huggins HA, Levy TE. Cerebrospinal fluid protein changes in multiple sclerosis after dental amalgam removal. Altern Med Rev 1998; 3(4):295-300. [ Google Scholar]
- Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 2001; 1(6):529-39. doi: 10.2174/1568026013394831 [Crossref] [ Google Scholar]
- Clausen J. Mercury and multiple sclerosis. Acta Neurol Scand 1993; 87(6):461-4. doi: 10.1111/j.1600-0404.1993.tb04137.x [Crossref] [ Google Scholar]
- Ingalls TH. Endemic clustering of multiple sclerosis in time and place, 1934-1984 Confirmation of a hypothesis. Am J Forensic Med Pathol 1986; 7(1):3-8. doi: 10.1097/00000433-198603000-00002 [Crossref] [ Google Scholar]
- Casetta I, Invernizzi M, Granieri E. Multiple sclerosis and dental amalgam: case-control study in Ferrara, Italy. Neuroepidemiology 2001; 20(2):134-7. doi: 10.1159/000054773 [Crossref] [ Google Scholar]
- Prochazkova J, Sterzl I, Kucerova H, Bartova J, Stejskal VD. The beneficial effect of amalgam replacement on health in patients with autoimmunity. Neuro Endocrinol Lett 2004; 25(3):211-8. [ Google Scholar]
- Chang LW, Hartmann HA. Ultrastructural studies of the nervous system after mercury intoxication I Pathological changes in the nerve cell bodies. Acta Neuropathol 1972; 20(2):122-38. doi: 10.1007/bf00691129 [Crossref] [ Google Scholar]
- Stejskal J, Stejskal VD. The role of metals in autoimmunity and the link to neuroendocrinology. Neuro Endocrinol Lett 1999; 20(6):351-64. [ Google Scholar]
- Issa Y, Watts DC, Duxbury AJ, Brunton PA, Watson MB, Waters CM. Mercuric chloride: toxicity and apoptosis in a human oligodendroglial cell line MO313. Biomaterials 2003; 24(6):981-7. doi: 10.1016/s0142-9612(02)00436-2 [Crossref] [ Google Scholar]
- Leong CC, Syed NI, Lorscheider FL. Retrograde degeneration of neurite membrane structural integrity of nerve growth cones following in vitro exposure to mercury. Neuroreport 2001; 12(4):733-7. doi: 10.1097/00001756-200103260-00024 [Crossref] [ Google Scholar]
- Chang LW. Neurotoxic effects of mercury--a review. Environ Res 1977; 14(3):329-73. doi: 10.1016/0013-9351(77)90044-5 [Crossref] [ Google Scholar]
- Bangsi D, Ghadirian P, Ducic S, Morisset R, Ciccocioppo S, McMullen E. Dental amalgam and multiple sclerosis: a case-control study in Montreal, Canada. Int J Epidemiol 1998; 27(4):667-71. doi: 10.1093/ije/27.4.667 [Crossref] [ Google Scholar]
- Madeddu R, Forte G, Bocca B, Tolu P, Sotgiu MA, Sotgiu G. Heavy metals and multiple sclerosis in Sardinian population (Italy). Anal Lett 2011; 44(9):1699-712. doi: 10.1080/00032719.2010.520396 [Crossref] [ Google Scholar]
- Faroon O, Ashizawa A, Wright S, Tucker P, Jenkins K, Ingerman L, et al. Toxicological Profile for Cadmium. Atlanta, GA: Agency for Toxic Substances and Disease Registry (US); 2012.
- Järup L, Hellström L, Alfvén T, Carlsson MD, Grubb A, Persson B. Low level exposure to cadmium and early kidney damage: the OSCAR study. Occup Environ Med 2000; 57(10):668-72. doi: 10.1136/oem.57.10.668 [Crossref] [ Google Scholar]
- Aliomrani M, Sahraian MA, Shirkhanloo H, Sharifzadeh M, Khoshayand MR, Ghahremani MH. Blood concentrations of cadmium and lead in multiple sclerosis patients from Iran. Iran J Pharm Res 2016; 15(4):825-33. [ Google Scholar]
- Tabatabaei SH, Nourmahnad N, Golestani Kermani S, Tabatabaei SA, Najafi P, Heidarpour M. Urban wastewater reuse in agriculture for irrigation in arid and semi-arid regions - a review. Int J Recycl Org Waste Agric 2020; 9(2):193-220. doi: 10.30486/ijrowa.2020.671672 [Crossref] [ Google Scholar]
- Arvidson B. Retrograde axonal transport of cadmium in the rat hypoglossal nerve. Neurosci Lett 1985; 62(1):45-9. doi: 10.1016/0304-3940(85)90282-4 [Crossref] [ Google Scholar]
- Kumar R, Agarwal AK, Seth PK. Oxidative stress-mediated neurotoxicity of cadmium. Toxicol Lett 1996; 89(1):65-9. doi: 10.1016/s0378-4274(96)03780-0 [Crossref] [ Google Scholar]
- Sinha M, Manna P, Sil PC. Cadmium-induced neurological disorders: prophylactic role of taurine. J Appl Toxicol 2008; 28(8):974-86. doi: 10.1002/jat.1363 [Crossref] [ Google Scholar]
- Renugadevi J, Prabu SM. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology 2009; 256(1-2):128-34. doi: 10.1016/j.tox.2008.11.012 [Crossref] [ Google Scholar]
- Shaikh ZA, Vu TT, Zaman K. Oxidative stress as a mechanism of chronic cadmium-induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicol Appl Pharmacol 1999; 154(3):256-63. doi: 10.1006/taap.1998.8586 [Crossref] [ Google Scholar]
- Aliomrani M, Sahraian MA, Shirkhanloo H, Sharifzadeh M, Khoshayand MR, Ghahremani MH. Correlation between heavy metal exposure and GSTM1 polymorphism in Iranian multiple sclerosis patients. Neurol Sci 2017; 38(7):1271-8. doi: 10.1007/s10072-017-2934-5 [Crossref] [ Google Scholar]
- Fulgenzi A, Zanella SG, Mariani MM, Vietti D, Ferrero ME. A case of multiple sclerosis improvement following removal of heavy metal intoxication: lessons learnt from Matteo’s case. Biometals 2012; 25(3):569-76. doi: 10.1007/s10534-012-9537-7 [Crossref] [ Google Scholar]
- Monroe RK, Halvorsen SW. Environmental toxicants inhibit neuronal Jak tyrosine kinase by mitochondrial disruption. Neurotoxicology 2009; 30(4):589-98. doi: 10.1016/j.neuro.2009.03.007 [Crossref] [ Google Scholar]
- Alizadeh-Ghodsi M, Zavvari A, Ebrahimi-Kalan A, Shiri-Shahsavar MR, Yousefi B. The hypothetical roles of arsenic in multiple sclerosis by induction of inflammation and aggregation of tau protein: a commentary. Nutr Neurosci 2018; 21(2):92-6. doi: 10.1080/1028415x.2016.1239399 [Crossref] [ Google Scholar]
- Bahrampour Juybari K, Ebrahimi G, Momeni Moghaddam MA, Asadikaram G, Torkzadeh-Mahani M, Akbari M. Evaluation of serum arsenic and its effects on antioxidant alterations in relapsing-remitting multiple sclerosis patients. Mult Scler Relat Disord 2018; 19:79-84. doi: 10.1016/j.msard.2017.11.010 [Crossref] [ Google Scholar]
- Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 2011; 31(2):95-107. doi: 10.1002/jat.1649 [Crossref] [ Google Scholar]
- Yousefi B, Ahmadi Y, Ghorbanihaghjo A, Faghfoori Z, Shafiei Irannejad V. Serum arsenic and lipid peroxidation levels in patients with multiple sclerosis. Biol Trace Elem Res 2014; 158(3):276-9. doi: 10.1007/s12011-014-9956-0 [Crossref] [ Google Scholar]
- Tsai CP, Lee CT. Multiple sclerosis incidence associated with the soil lead and arsenic concentrations in Taiwan. PLoS One 2013; 8(6):e65911. doi: 10.1371/journal.pone.0065911 [Crossref] [ Google Scholar]
- Nazıroğlu M, Yürekli VA. Effects of antiepileptic drugs on antioxidant and oxidant molecular pathways: focus on trace elements. Cell Mol Neurobiol 2013; 33(5):589-99. doi: 10.1007/s10571-013-9936-5 [Crossref] [ Google Scholar]
- De Vizcaya-Ruiz A, Barbier O, Ruiz-Ramos R, Cebrian ME. Biomarkers of oxidative stress and damage in human populations exposed to arsenic. Mutat Res 2009; 674(1-2):85-92. doi: 10.1016/j.mrgentox.2008.09.020 [Crossref] [ Google Scholar]
- Smith KJ. Newly lesioned tissue in multiple sclerosis--a role for oxidative damage?. Brain 2011; 134(Pt 7):1877-81. doi: 10.1093/brain/awr144 [Crossref] [ Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007; 87(1):245-313. doi: 10.1152/physrev.00044.2005 [Crossref] [ Google Scholar]
- Islam MT. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res 2017; 39(1):73-82. doi: 10.1080/01616412.2016.1251711 [Crossref] [ Google Scholar]
- Dehghanifiroozabadi M, Noferesti P, Amirabadizadeh A, Nakhaee S, Aaseth J, Noorbakhsh F. Blood lead levels and multiple sclerosis: a case-control study. Mult Scler Relat Disord 2019; 27:151-5. doi: 10.1016/j.msard.2018.10.010 [Crossref] [ Google Scholar]
- Forte G, Madeddu R, Tolu P, Asara Y, Marchal JA, Bocca B. Reference intervals for blood Cd and Pb in the general population of Sardinia (Italy). Int J Hyg Environ Health 2011; 214(2):102-9. doi: 10.1016/j.ijheh.2010.09.006 [Crossref] [ Google Scholar]
- Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011; 283(2-3):65-87. doi: 10.1016/j.tox.2011.03.001 [Crossref] [ Google Scholar]
- Bhat SA, Hassan T, Majid S. Heavy metal toxicity and their harmful effects on living organisms–a review. Int J Med Sci Diag Res 2019; 3(1):106-12. [ Google Scholar]
- Komárek M, Ettler V, Chrastný V, Mihaljevic M. Lead isotopes in environmental sciences: a review. Environ Int 2008; 34(4):562-77. doi: 10.1016/j.envint.2007.10.005 [Crossref] [ Google Scholar]
- González-Estecha M, Trasobares E, Fuentes M, Martínez MJ, Cano S, Vergara N. Blood lead and cadmium levels in a six hospital employee population PESA study, 2009. J Trace Elem Med Biol 2011; 25 Suppl 1:S22-9. doi: 10.1016/j.jtemb.2010.10.004 [Crossref] [ Google Scholar]
- Kalia K, Flora SJ. Strategies for safe and effective therapeutic measures for chronic arsenic and lead poisoning. J Occup Health 2005; 47(1):1-21. doi: 10.1539/joh.47.1 [Crossref] [ Google Scholar]
- The Birmingham Research Unit of the Royal College of General Practitioners and the Department of Social Medicine, University of Birmingham. Lead and multiple sclerosis. J R Coll Gen Pract. 1976;26(169):622-6.
- Brito JA, Costa IM, e Silva AM, Marques JM, Zagalo CM, Cavaleiro II. Changes in bone Pb accumulation: cause and effect of altered bone turnover. Bone 2014; 64:228-34. doi: 10.1016/j.bone.2014.04.021 [Crossref] [ Google Scholar]
- Etemadifar M, Mehrabi B, Kiani-Peykani R, Abtahi SH, Nekouie-Isfahani K, Ramagopalan SV. Soil heavy metals are associated with the distribution of multiple sclerosis in Isfahan, Iran. Acta Neurol Scand 2016; 134(4):292-9. doi: 10.1111/ane.12543 [Crossref] [ Google Scholar]
- Amirinejad R, Shirvani-Farsani Z, Naghavi Gargari B, Sahraian MA, Mohammad Soltani B, Behmanesh M. Vitamin D changes expression of DNA repair genes in the patients with multiple sclerosis. Gene 2021; 781:145488. doi: 10.1016/j.gene.2021.145488 [Crossref] [ Google Scholar]
- Cortese M, Munger KL, Martínez-Lapiscina EH, Barro C, Edan G, Freedman MS. Vitamin D, smoking, EBV, and long-term cognitive performance in MS: 11-year follow-up of BENEFIT. Neurology 2020; 94(18):e1950-e60. doi: 10.1212/wnl.0000000000009371 [Crossref] [ Google Scholar]
- Mazumdar I, Goswami K, Ali MS. Status of serum calcium, vitamin D and parathyroid hormone and hematological indices among lead exposed jewelry workers in Dhaka, Bangladesh. Indian J Clin Biochem 2017; 32(1):110-6. doi: 10.1007/s12291-016-0582-9 [Crossref] [ Google Scholar]
- Rahman A, Al-Awadi AA, Khan KM. Lead affects vitamin D metabolism in rats. Nutrients 2018; 10(3):264. doi: 10.3390/nu10030264 [Crossref] [ Google Scholar]
- Warren HV, Delavault RE, Cross CH. Possible correlations between geology and some disease patterns. Ann N Y Acad Sci 1967; 136(22):657-710. doi: 10.1111/j.1749-6632.1967.tb39685.x [Crossref] [ Google Scholar]
- Warren HV. Proceedings: environmental lead: a survey of its possible physiological significance. J Biosoc Sci 1974; 6(2):223-38. [ Google Scholar]
- Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 1995; 18(2):321-36. doi: 10.1016/0891-5849(94)00159-h [Crossref] [ Google Scholar]
- Patrick L. Lead toxicity, a review of the literature Part 1: exposure, evaluation, and treatment. Altern Med Rev 2006; 11(1):2-22. [ Google Scholar]
- Sinicropi MS, Amantea D, Caruso A, Saturnino C. Chemical and biological properties of toxic metals and use of chelating agents for the pharmacological treatment of metal poisoning. Arch Toxicol 2010; 84(7):501-20. doi: 10.1007/s00204-010-0544-6 [Crossref] [ Google Scholar]
- Migliore L, Coppedè F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat Res 2009; 674(1-2):73-84. doi: 10.1016/j.mrgentox.2008.09.013 [Crossref] [ Google Scholar]
- Mishra KP. Lead exposure and its impact on immune system: a review. Toxicol In Vitro 2009; 23(6):969-72. doi: 10.1016/j.tiv.2009.06.014 [Crossref] [ Google Scholar]
- Forte G, Visconti A, Santucci S, Ghazaryan A, Figà-Talamanca L, Cannoni S. Quantification of chemical elements in blood of patients affected by multiple sclerosis. Ann Ist Super Sanita 2005; 41(2):213-6. [ Google Scholar]
- Razavi Z, Jokar M, Allafchian A, Hossinpour Z, Berenjani L, Shayegan Nejad V. The relationship between blood lead levels and clinical features among multiple sclerosis patients in Isfahan, Iran. Iran J Health Saf Environ 2016; 3(1):412-20. [ Google Scholar]
- Ghoreishi A, Mohseni M, Amraei R, Mirza Alizadeh A, Mazloomzadeh S. Investigation the amount of copper, lead, zinc and cadmium levels in serum of Iranian multiple sclerosis patients. J Chem Pharm Sci 2015; 8(1):40-5. [ Google Scholar]
- Bell JS, DeLuca GC. Genes, smoking, and organic solvent exposure: an alarming cocktail for MS risk. Neurology 2018; 91(5):199-200. doi: 10.1212/wnl.0000000000005896 [Crossref] [ Google Scholar]
- Gourley M, Miller FW. Mechanisms of disease: environmental factors in the pathogenesis of rheumatic disease. Nat Clin Pract Rheumatol 2007; 3(3):172-80. doi: 10.1038/ncprheum0435 [Crossref] [ Google Scholar]
- White RF, Proctor SP. Solvents and neurotoxicity. Lancet 1997; 349(9060):1239-43. doi: 10.1016/s0140-6736(96)07218-2 [Crossref] [ Google Scholar]
- Ketterer B, Coles B, Meyer DJ. The role of glutathione in detoxication. Environ Health Perspect 1983; 49:59-69. doi: 10.1289/ehp.834959 [Crossref] [ Google Scholar]
- Landtblom AM, Wastenson M, Ahmadi A, Söderkvist P. Multiple sclerosis and exposure to organic solvents, investigated by genetic polymorphisms of the GSTM1 and CYP2D6 enzyme systems. Neurol Sci 2003; 24(4):248-51. doi: 10.1007/s10072-003-0148-5 [Crossref] [ Google Scholar]
- Saghafi Z, Mohammadi M, Mahboobian MM, Derakhshandeh K. Preparation, characterization, and in vivo evaluation of perphenazine-loaded nanostructured lipid carriers for oral bioavailability improvement. Drug Dev Ind Pharm 2021; 47(3):509-20. doi: 10.1080/03639045.2021.1892745 [Crossref] [ Google Scholar]
- Mohammadi M, Elahimehr Z, Mahboobian MM. Acyclovir-loaded nanoemulsions: preparation, characterization and irritancy studies for ophthalmic delivery. Curr Eye Res 2021; 46(11):1646-52. doi: 10.1080/02713683.2021.1929328 [Crossref] [ Google Scholar]
- Samimi MS, Mahboobian MM, Mohammadi M. Ocular toxicity assessment of nanoemulsion in-situ gel formulation of fluconazole. Hum Exp Toxicol 2021; 40(12):2039-47. doi: 10.1177/09603271211017314 [Crossref] [ Google Scholar]
- Talaei S, Mahboobian MM, Mohammadi M. Investigating the ocular toxicity potential and therapeutic efficiency of in situ gel nanoemulsion formulations of brinzolamide. Toxicol Res (Camb) 2020; 9(4):578-87. doi: 10.1093/toxres/tfaa066 [Crossref] [ Google Scholar]
- Al-Hajri Z, Del Bigio MR. Brain damage in a large cohort of solvent abusers. Acta Neuropathol 2010; 119(4):435-45. doi: 10.1007/s00401-010-0653-6 [Crossref] [ Google Scholar]
- Flodin U, Landtblom AM, Axelson O. Multiple sclerosis in nurse anaesthetists. Occup Environ Med 2003; 60(1):66-8. doi: 10.1136/oem.60.1.66 [Crossref] [ Google Scholar]
- Amaducci L, Arfaioli C, Inzitari D, Marchi M. Multiple sclerosis among shoe and leather workers: an epidemiological survey in Florence. Acta Neurol Scand 1982; 65(2):94-103. doi: 10.1111/j.1600-0404.1982.tb03066.x [Crossref] [ Google Scholar]
- Landtblom AM, Flodin U, Karlsson M, Pålhagen S, Axelson O, Söderfeldt B. Multiple sclerosis and exposure to solvents, ionizing radiation and animals. Scand J Work Environ Health 1993; 19(6):399-404. doi: 10.5271/sjweh.1455 [Crossref] [ Google Scholar]
- Söderkvist P, Ahmadi A, Akerbäck A, Axelson O, Flodin U. Glutathione S-transferase M1 null genotype as a risk modifier for solvent-induced chronic toxic encephalopathy. Scand J Work Environ Health 1996; 22(5):360-3. doi: 10.5271/sjweh.154 [Crossref] [ Google Scholar]
- Reis J, Dietemann JL, Warter JM, Poser CM. A case of multiple sclerosis triggered by organic solvents. Neurol Sci 2001; 22(2):155-8. doi: 10.1007/s100720170015 [Crossref] [ Google Scholar]
- Talhout R, Schulz T, Florek E, van Benthem J, Wester P, Opperhuizen A. Hazardous compounds in tobacco smoke. Int J Environ Res Public Health 2011; 8(2):613-28. doi: 10.3390/ijerph8020613 [Crossref] [ Google Scholar]
- Kalra R, Singh SP, Savage SM, Finch GL, Sopori ML. Effects of cigarette smoke on immune response: chronic exposure to cigarette smoke impairs antigen-mediated signaling in T cells and depletes IP3-sensitive Ca2 + stores. J Pharmacol Exp Ther 2000; 293(1):166-71. [ Google Scholar]
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol 2003; 43:309-34. doi: 10.1146/annurev.pharmtox.43.100901.135828 [Crossref] [ Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 2008; 453(7191):106-9. doi: 10.1038/nature06881 [Crossref] [ Google Scholar]
- Duarte JH, Di Meglio P, Hirota K, Ahlfors H, Stockinger B. Differential influences of the aryl hydrocarbon receptor on Th17 mediated responses in vitro and in vivo. PLoS One 2013; 8(11):e79819. doi: 10.1371/journal.pone.0079819 [Crossref] [ Google Scholar]
- Garey KW, Neuhauser MM, Robbins RA, Danziger LH, Rubinstein I. Markers of inflammation in exhaled breath condensate of young healthy smokers. Chest 2004; 125(1):22-6. doi: 10.1378/chest.125.1.22 [Crossref] [ Google Scholar]
- Bermudez EA, Rifai N, Buring JE, Manson JE, Ridker PM. Relation between markers of systemic vascular inflammation and smoking in women. Am J Cardiol 2002; 89(9):1117-9. doi: 10.1016/s0002-9149(02)02284-1 [Crossref] [ Google Scholar]
- Tracy RP, Psaty BM, Macy E, Bovill EG, Cushman M, Cornell ES. Lifetime smoking exposure affects the association of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. Arterioscler Thromb Vasc Biol 1997; 17(10):2167-76. doi: 10.1161/01.atv.17.10.2167 [Crossref] [ Google Scholar]
- Hughes DA, Haslam PL, Townsend PJ, Turner-Warwick M. Numerical and functional alterations in circulatory lymphocytes in cigarette smokers. Clin Exp Immunol 1985; 61(2):459-66. [ Google Scholar]
- Moszczyński P, Zabiński Z, Moszczyński P Jr, Rutowski J, Słowiński S, Tabarowski Z. Immunological findings in cigarette smokers. Toxicol Lett 2001; 118(3):121-7. doi: 10.1016/s0378-4274(00)00270-8 [Crossref] [ Google Scholar]
- Hersey P, Prendergast D, Edwards A. Effects of cigarette smoking on the immune system Follow-up studies in normal subjects after cessation of smoking. Med J Aust 1983; 2(9):425-9. [ Google Scholar]
- Philbrick DJ, Hopkins JB, Hill DC, Alexander JC, Thomson RG. Effects of prolonged cyanide and thiocyanate feeding in rats. J Toxicol Environ Health 1979; 5(4):579-92. doi: 10.1080/15287397909529770 [Crossref] [ Google Scholar]
- Pryor WA, Stone K. Oxidants in cigarette smoke Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci 1993; 686:12-27. doi: 10.1111/j.1749-6632.1993.tb39148.x [Crossref] [ Google Scholar]
- Rejdak K, Eikelenboom MJ, Petzold A, Thompson EJ, Stelmasiak Z, Lazeron RH. CSF nitric oxide metabolites are associated with activity and progression of multiple sclerosis. Neurology 2004; 63(8):1439-45. doi: 10.1212/01.wnl.0000142043.32578.5d [Crossref] [ Google Scholar]
- Mawatari S. Biochemical study on rat brain in acute carbon monoxide poisoning. Folia Psychiatr Neurol Jpn 1970; 24(2):123-9. doi: 10.1111/j.1440-1819.1970.tb01465.x [Crossref] [ Google Scholar]
- Hans FJ, Wei L, Bereczki D, Acuff V, Demaro J, Chen JL. Nicotine increases microvascular blood flow and flow velocity in three groups of brain areas. Am J Physiol 1993; 265(6 Pt 2):H2142-50. doi: 10.1152/ajpheart.1993.265.6.H2142 [Crossref] [ Google Scholar]
- Handel AE, Williamson AJ, Disanto G, Dobson R, Giovannoni G, Ramagopalan SV. Smoking and multiple sclerosis: an updated meta-analysis. PLoS One 2011; 6(1):e16149. doi: 10.1371/journal.pone.0016149 [Crossref] [ Google Scholar]
- Brønnum-Hansen H, Koch-Henriksen N, Stenager E. Trends in survival and cause of death in Danish patients with multiple sclerosis. Brain 2004; 127(Pt 4):844-50. doi: 10.1093/brain/awh104 [Crossref] [ Google Scholar]
- Manouchehrinia A, Weston M, Tench CR, Britton J, Constantinescu CS. Tobacco smoking and excess mortality in multiple sclerosis: a cohort study. J Neurol Neurosurg Psychiatry 2014; 85(10):1091-5. doi: 10.1136/jnnp-2013-307187 [Crossref] [ Google Scholar]
- Manouchehrinia A, Tanasescu R, Tench CR, Constantinescu CS. Mortality in multiple sclerosis: meta-analysis of standardised mortality ratios. J Neurol Neurosurg Psychiatry 2016; 87(3):324-31. doi: 10.1136/jnnp-2015-310361 [Crossref] [ Google Scholar]
- van der Vuurst de Vries RM, Mescheriakova JY, Runia TF, Siepman TAM, Wokke BHA, Samijn JPA. Smoking at time of CIS increases the risk of clinically definite multiple sclerosis. J Neurol 2018; 265(5):1010-5. doi: 10.1007/s00415-018-8780-4 [Crossref] [ Google Scholar]
- Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P. An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater 2018; 344:179-99. doi: 10.1016/j.jhazmat.2017.10.014 [Crossref] [ Google Scholar]
- de La Mantia F. Handbook of Plastics Recycling. Shrewsbury, UK: Smithers Rapra Technology; 2002.
- Arvanitoyannis IS, Bosnea L. Migration of substances from food packaging materials to foods. Crit Rev Food Sci Nutr 2004; 44(2):63-76. doi: 10.1080/10408690490424621 [Crossref] [ Google Scholar]
- Goodson A, Robin H, Summerfield W, Cooper I. Migration of bisphenol A from can coatings--effects of damage, storage conditions and heating. Food Addit Contam 2004; 21(10):1015-26. doi: 10.1080/02652030400011387 [Crossref] [ Google Scholar]
- Ibrahim MZ, Reder AT, Lawand R, Takash W, Sallouh-Khatib S. The mast cells of the multiple sclerosis brain. J Neuroimmunol 1996; 70(2):131-8. doi: 10.1016/s0165-5728(96)00102-6 [Crossref] [ Google Scholar]
- Krüger PG. Mast cells: the key to multiple sclerosis?. World J Neurosci 2014; 4(2):45101. doi: 10.4236/wjns.2014.42014 [Crossref] [ Google Scholar]
- Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaie A. Pesticides and oxidative stress: a review. Med Sci Monit 2004; 10(6):RA141-7. [ Google Scholar]
- Banerjee BD, Seth V, Bhattacharya A, Pasha ST, Chakraborty AK. Biochemical effects of some pesticides on lipid peroxidation and free-radical scavengers. Toxicol Lett 1999; 107(1-3):33-47. doi: 10.1016/s0378-4274(99)00029-6 [Crossref] [ Google Scholar]
- Ataei S, Abaspanah S, Haddadi R, Mohammadi M, Nili-Ahmadabadi A. Therapeutic potential of dihydropyridine calcium channel blockers on oxidative injury caused by organophosphates in cortex and cerebellum: an in vivo study. Indian J Clin Biochem 2020; 35(3):339-46. doi: 10.1007/s12291-019-00830-3 [Crossref] [ Google Scholar]
- Pathak R, Suke SG, Ahmed T, Ahmed RS, Tripathi AK, Guleria K. Organochlorine pesticide residue levels and oxidative stress in preterm delivery cases. Hum Exp Toxicol 2010; 29(5):351-8. doi: 10.1177/0748233710363334 [Crossref] [ Google Scholar]
- Kori RK, Singh MK, Jain AK, Yadav RS. Neurochemical and behavioral dysfunctions in pesticide exposed farm workers: a clinical outcome. Indian J Clin Biochem 2018; 33(4):372-81. doi: 10.1007/s12291-018-0791-5 [Crossref] [ Google Scholar]
- Yadav RS, Tiwari NK. Lipid integration in neurodegeneration: an overview of Alzheimer’s disease. Mol Neurobiol 2014; 50(1):168-76. doi: 10.1007/s12035-014-8661-5 [Crossref] [ Google Scholar]
- Yadav SS, Singh MK, Yadav RS. Organophosphates induced Alzheimer’s disease: an epigenetic aspect. J Clin Epigenet 2016; 2(1):1-8. [ Google Scholar]
- Magyari M, Koch-Henriksen N, Pfleger CC, Sørensen PS. Gender and autoimmune comorbidity in multiple sclerosis. Mult Scler 2014; 20(9):1244-51. doi: 10.1177/1352458514521515 [Crossref] [ Google Scholar]
- Parrón T, Requena M, Hernández AF, Alarcón R. Association between environmental exposure to pesticides and neurodegenerative diseases. Toxicol Appl Pharmacol 2011; 256(3):379-85. doi: 10.1016/j.taap.2011.05.006 [Crossref] [ Google Scholar]
- Graves JS, Chitnis T, Weinstock-Guttman B, Rubin J, Zelikovitch AS, Nourbakhsh B. Maternal and perinatal exposures are associated with risk for pediatric-onset multiple sclerosis. Pediatrics 2017; 139(4):e20162838. doi: 10.1542/peds.2016-2838 [Crossref] [ Google Scholar]
- Menini T, Gugliucci A. Paraoxonase 1 in neurological disorders. Redox Rep 2014; 19(2):49-58. doi: 10.1179/1351000213y.0000000071 [Crossref] [ Google Scholar]
- Dwivedi N, Flora SJ. Concomitant exposure to arsenic and organophosphates on tissue oxidative stress in rats. Food Chem Toxicol 2011; 49(5):1152-9. doi: 10.1016/j.fct.2011.02.007 [Crossref] [ Google Scholar]
- Latchoumycandane C, Chitra KC, Mathur PP. The effect of methoxychlor on the epididymal antioxidant system of adult rats. Reprod Toxicol 2002; 16(2):161-72. doi: 10.1016/s0890-6238(02)00002-3 [Crossref] [ Google Scholar]
- Gultekin F, Ozturk M, Akdogan M. The effect of organophosphate insecticide chlorpyrifos-ethyl on lipid peroxidation and antioxidant enzymes (in vitro). Arch Toxicol 2000; 74(9):533-8. doi: 10.1007/s002040000167 [Crossref] [ Google Scholar]
- Gupta RC, Milatovic D, Dettbarn WD. Depletion of energy metabolites following acetylcholinesterase inhibitor-induced status epilepticus: protection by antioxidants. Neurotoxicology 2001; 22(2):271-82. doi: 10.1016/s0161-813x(01)00013-4 [Crossref] [ Google Scholar]
- Bayoumi AE, Pérez-Pertejo Y, Ordóñez C, Reguera RM, Balaña-Fouce R, Ordóñez D. Changes in the glutathione-redox balance induced by the pesticides heptachlor, chlordane, and toxaphene in CHO-K1 cells. Bull Environ Contam Toxicol 2000; 65(6):748-55. doi: 10.1007/s0012800186 [Crossref] [ Google Scholar]
- Bachowski S, Xu Y, Stevenson DE, Walborg EF Jr, Klaunig JE. Role of oxidative stress in the selective toxicity of dieldrin in the mouse liver. Toxicol Appl Pharmacol 1998; 150(2):301-9. doi: 10.1006/taap.1998.8372 [Crossref] [ Google Scholar]
- Hincal F, Gürbay A, Giray B. Induction of lipid peroxidation and alteration of glutathione redox status by endosulfan. Biol Trace Elem Res 1995; 47(1-3):321-6. doi: 10.1007/bf02790133 [Crossref] [ Google Scholar]
- Numan IT, Hassan MQ, Stohs SJ. Endrin-induced depletion of glutathione and inhibition of glutathione peroxidase activity in rats. Gen Pharmacol 1990; 21(5):625-8. doi: 10.1016/0306-3623(90)91008-f [Crossref] [ Google Scholar]