Avicenna Journal of Pharmaceutical Research. :23-31.
doi: 10.34172/ajpr.2022.1055
Original Article
Decreased Interleukin 6 and Tumor Necrosis Factor α Proteins and Metalloproteinase-2,9 Levels Following Cerebral Ischemia in Cinnamon-treated Rats
Mohammad Reza Mostajabi 1, Maryam Tavabi 1, Massoud Hatami 1, Mahin Ghanjkhani 1, Seyyed Saeid Mousavi 2, Mojtaba Fathi 3, Mehdi Eskandari 1, Hossein Mostafavi 1, *  , Meysam Forouzandeh 4, Elham Ghasemloo 1
, Meysam Forouzandeh 4, Elham Ghasemloo 1
Author information:
1Department of Physiology, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
2Animal Science Research Department, Zanjan Agricultural and Natural Resources Research and Education Center, AREEO, Zanjan, Iran
3Department of Biochemistry, Medical School, Zanjan University of Medical Sciences, Zanjan, Iran
4Faculty of Life Sciences, Shahid-Beheshti University, Tehran, Iran
Abstract
Background: Metabolic disturbances, including hyperlipidaemia, are risk factors for brain ischemia. Despite all research, no definitive treatment has yet been found for brain ischemia. Thus, this study investigated the effect of cinnamon on ischemic tolerance and the expression of matrix metalloproteinase (MMP) 2 and 9 genes, as well as levels of interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α) proteins in rats receiving a high-fat diet (HFD).
Methods: Rats were divided into control, sham, model, lovastatin, vehicle, and cinnamon 130 mg (Cin130) and 260 mg (Cin260). All groups, except for the control group, received a high-fat diet for 6 weeks. Then, the last four groups received the corresponding treatment for 6 weeks. The stroke was induced by middle cerebral artery occlusion (MCAO). Twelve hours later, the animals were evaluated for the extent of neurological defects, lipid profiles, tissue damage, and gene expression of MMP-2,9, as well as IL-6 and TNF-α protein level.
Results: The results showed that Cin130 has been highly successful in improving neurological defects. Cin130 was effective in reducing serum cholesterol and triglyceride. Further, Cin260 effectively reduced serum LDL. Moreover, Cin130 and Cin260 could reduce tissue damage volume, blood-brain barrier (BBB) integrity, and down-regulation of IL-6, TNF-α proteins and MMP-2,9 levels.
Conclusion: Overall, cinnamon, in addition to its lipid-lowering effect, may also have a neuroprotective effect that may be related to the down-regulation of MMPs and cytokines. The two may be independent events or related to a third event; in any case, appropriate experiments should be performed in future studies to determine whether altering the MMPs reduces the effectiveness of cinnamon.
Keywords: Hyperlipidaemia, Brain Ischemia, Matrix metalloproteinase, Interleukin-6
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Mostajabi MR, Tavabi M, Hatami M, Ghanjkhani M, Mousavi SS, Fathi M, et al. Decreased interleukin 6 and tumor necrosis factor α proteins and metalloproteinase-2,9 levels following cerebral ischemia in cinnamon-treated rats. Avicenna J Pharm Res. 2022; 3(1):23-31. doi:10.34172/ajpr.2022.1055
Introduction
Ischemia is a cerebrovascular disorder that occurs due to abnormal blood flow to the part of the brain. It is the third leading cause of death, after cancer and cardiovascular diseases, in industrialized countries. Most studies have aimed at the prevention and treatment of ischemic stroke. One of the events following ischemia is the reperfusion of the ischemic region, which not only cannot restore the condition but also can worsen the condition (1,2). According to previous studies, large-artery atherosclerosis was the most common cause of ischemia in middle-aged patients (3,4). Investigations have shown that a high-fat diet (HFD) was accompanied by increased cerebrovascular changes which increased infarct size in the animal model of ischemia (5,6). In addition, based on the report of Hoane et al in the animal model of a cortical contusion injury, HFD caused significantly more excessive loss of cortical tissue (7). Eid et al also showed that an HFD is associated with oxidative stress (OS) and mitochondria-mediated cell death (8). Changes made after ischemia-reperfusion (I/R) include the production of free radicals (9), cell membrane lipid peroxidation, activation of cyclooxygenase 2 (COX-2) and nitric oxide synthase -2 enzymes, expression of inflammatory factors, blood-brain barrier (BBB) failure, infiltration of inflammatory factors into damaged tissue, activation of matrix metalloproteinases (MMPs) (10,11), DNA damage, and finally cell death (12).
Hyperlipidemia and associated atherosclerosis are among the main preventable risk factors for stroke. Approximately two-thirds of deaths from atherosclerosis are due to one or more coronary artery thrombosis, and one-third remain due to thrombosis or bleeding of the veins in the other organs of the body, especially the brain, which causes hemorrhagic and ischemic stroke (13,14).
Some studies have demonstrated that following neurological disorders, including cerebral ischemia (15) and Parkinson’s disease (16), the expression level of inflammatory factors, including interleukin 6 (IL-6), tumor necrosis factor α (TNF-α), and MMP-2,9 increases, leading to increased BBB permeability by damaging tight junction proteins, thus exacerbating the complications of cerebral ischemia, including tissue damage, edema, and neurological defects. It was also found that damage to BBB is more severe in rats receiving an HFD compared to rats receiving a normal diet. Hyperlipidemia results in increased inflammatory responses during cerebral ischemia, including the accelerated release of proinflammatory cytokines such as IL-6, TNF-α, and an increase in the expression level of MMP-2,9. These findings suggest that there is a close association between hyperlipidemia and exacerbation of inflammation and MMP-2,9 activity, ultimately resulting in more severe damage to BBB and exacerbation of ischemic injuries (17). Therefore, the use of interventions that are effective in controlling lipid profile while simultaneously reducing inflammation and the expression level of MMPs and thus strengthening BBB integrity, has a significant effect in reducing the complications of ischemia and the extent of tissue damage.
Taking into account the concerns of people about the side effects of chemical drugs, today, the use of herbal compounds with different properties is widely considered in the medical sciences (18). Medicinal plants are among the most important sources of blood lipid-lowering agents. Cinnamomum zeylanicum, with the general name of cinnamon, has a long history of use as spice, flavoring agent, and preservative. Several studies have confirmed its beneficial effects in the treatment of many diseases, including hyperlipidemia (19,20). So far, several properties of cinnamon have been reported, including hypolipidemic effects (21), anti-inflammatory, antioxidant (22), neuroprotective (23), anti-diabetic (24), and thrombolytic properties (25).
Considering that most of the studies focused on the effect of cinnamon on I/R in healthy and non-hyperlipidemic rats, this study aimed to investigate the effect of the cinnamon hydroalcoholic extract on ischemic tolerance by the modification of lipid profile and expression of MMP-2,9 genes and IL-6 and TNF-α protein levels in rats receiving an HFD. Given that previous studies have represented thelipid-lowering and neuroprotective properties of lovastatin (25,26), the present study has used this drug as a positive control to compare the effects of cinnamon.
Materials and Methods
Preparation of an HFD
The HFD was prepared according to previous articles with some modifications (27,28). Briefly, the usual meal was molded, and 200 g of the sheep’s fat was added for every 800 g of the powdered food. Distilled water was added to the powdered food for dough, and it was completely mixed by kneading. This dough mixture was converted into a pellet form and then laid on racks for drying. The total drying time was 36 hours. The dried strands of the pellet were broken into short lengths and stored in the refrigerator. This meal was prepared weekly.
Preparation of the Extract of Cinnamon
The hydroalcoholic cinnamon extract and lovastatin powder were obtained from Golestan Company (Tehran, Iran) and Osveh Pharmaceutical Company (Tehran, Iran), respectively. According to the recommended dosage in the articles, 10 mg of the powder per kg body weight of the animal was used (29), and carboxymethylcellulose (CMC) 0.5% was employed as a solvent for lovastatin powder (30).
Animals
Overall, 84 adult male Wistar rats weighing 180-220 g with an age range of approximately 6-8 weeks (obtained from the Animal Laboratory of Zanjan University of Medical Sciences) were included in this research. Rats were kept in appropriate bioclimatic conditions, including 12 hours of light, 12 hours of darkness, 22-24 °C, and 60% humidity. To adapt to the environment, the animals were transferred to the laboratory for one week before the study. During this time, the animals had free access to water and food. All experiments were performed at 2 PM and according to the Laboratory Animal Health Care and Use Guidelines of Zanjan University of Medical Sciences. This study was conducted in compliance with the ethical guidelines of Zanjan University of Medical Sciences (Ethical No: ZUMS.REC.1396.75).
The animals were randomly divided into seven groups of 12, including the control, sham, stroke model, lovastatin, vehicle, low-dose cinnamon, and high-dose cinnamon groups.
Animals in the control group received the usual diet. Those in the sham group received an HFD for eight weeks and underwent surgical stress in the 12th week. Further, the animals of the stroke model group received an HFD for eight weeks and underwent middle cerebral artery occlusion (MCAO) surgery at week 12. Furthermore, the lovastatin group received an HFD for eight weeks; then, it received 10 mg/kg lovastatin (29) for 4 weeks by the intraperitoneal (IP) injection and underwent MCAO surgery at week 12. Moreover, animals in the vehicle group received an HFD for eight weeks. Next, they received 0.5% carboxymethyl cellulase for 4 weeks by the IP injection and underwent MCAO surgery at week 12. Additionally, the low-dose cinnamon group received an HFD for eight weeks. Then, each animal received 130 mg of the hydroalcoholic extract of cinnamon (31) by the IP injection for four weeks and underwent MCAO surgery at week 12. Finally, the animals of the high-dose cinnamon group received an HFD for eight weeks; then, they received 260 mg of the hydroalcoholic extract of cinnamon by the IP injection for four weeks and underwent MCAO surgery at week 12. The related data are depicted in Figure 1.
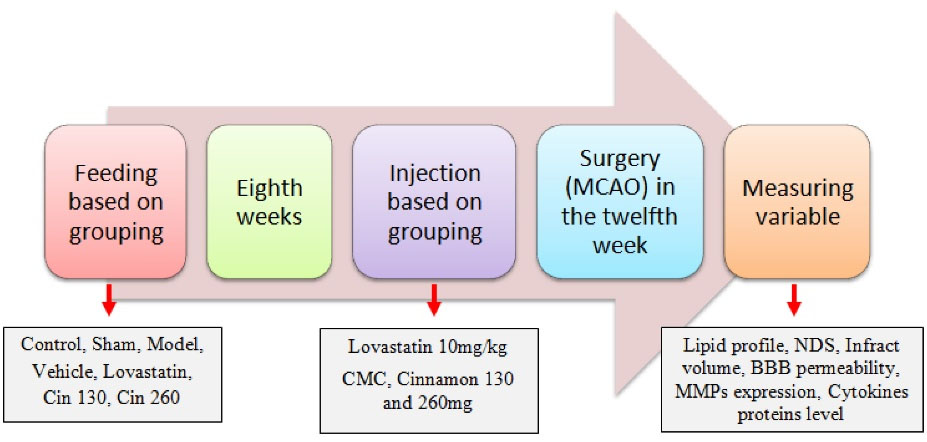
Figure 1.
Schematic Diagram of the Experimental Design. Note. CMC: Carboxymethylcellulose, NDS: Neurologic deficit scores
.
Schematic Diagram of the Experimental Design. Note. CMC: Carboxymethylcellulose, NDS: Neurologic deficit scores
Induction of Stroke With MCAO
The MCAO was inducted according to the intraluminal filament model (32). Briefly, rats were anesthetized using ketamine (60 mg/kg) and xylazine (10 mg/kg) through IP injection. The right common carotid artery was isolated, and silicone-coated nylon was inserted, along the right common carotid arteries, into the internal carotid artery until it blocked the origin of the middle cerebral artery. The depth of penetration was approximately 20 mm from the carotid bifurcation to effectively block the middle cerebral artery. Rats were subjected to focal ischemia for 60 minutes; blood flow was restored by withdrawing the nylon filament. The surgery was performed at 37 ± 0.3°C. After recovery from anesthesia, the animals were returned to their cages.
Neurological Examination
A neurological score was used to evaluate the achievement of the model. Neurological function was investigated using a 0-5 point scale neurological score (33) after 12 hours of reperfusion. The scores on the scale ranged from 0 = No neurological dysfunction, 1 = Failure to extend opposite forepaw, 2 = Circling to the contralateral side when held by the tail with feet on the floor, 3 = Falling to the left, unable to bear weight on the affected side, 4 = No spontaneous walking and a depressed level of consciousness to 5 = Death.
Blood Sampling
According to the report of Liu and McCullough, the entire territory supplied by the MCA is damaged 12 hours after MCAO (34). Therefore, 12 hours after the induction of ischemia, the animals were anesthetized with chloral hydrate, and then the blood was taken from the heart. The blood samples were centrifuged at 7000 × g for 10 minutes at room temperature to obtain the serum. The serum samples were stored at -20°C until biochemical analysis. The serum levels of cholesterol, triglyceride, and high-density lipoprotein (HDL) were measured by spectrophotometry (enzymatic colorimetry) and using special measuring kits (Pars Azmon). Low-density lipoprotein (LDL) levels were obtained by the following formula:
LDL (mg/dL) = Total cholesterol - (HDL + TG/5)
2, 3, 5-Triphenyl-2-tetrazolium Chloride (TTC) Staining
TTC staining was performed for measuring the infarct volume. The brains of the rats were rapidly removed after the decapitation of the animals under a high dose of chloral hydrate anesthesia (800 mg/kg). The brains were cooled in ice-cold saline for 10 minutes and then sliced into 2-mm-thick coronal sections starting from the forebrain area using a brain matrix slicer. Brain slices were incubated for 20 minutes in a 2% solution of TTC (Merk, Germany) and kept at 37°C in a water bath for 15 minutes. Next, healthy tissues became red in color due to the presence of succinate dehydrogenase, and the lesioned tissue that did not have this enzyme remained white. Then, the image was taken from the sections. After transferring the images to the computer, the volume of the stroke was calculated using the ImageJ software. The corrected infarct volume was obtained based on the following formula (15):
Corrected infarct volume = Left hemisphere volume-(right hemisphere volume - infarct volume).
Measurement of BBB Permeability
The BBB integrity was evaluated by measuring Evans Blue (EB) extravasations. After 60 minutes of ischemia, 4 mL/kg of 2% EB solution was injected through the animal’s tail vein. The rats were anesthetized 12 hours after reperfusion and the use of the transcardial technique, 250 mL of the saline was infused through the left ventricle to remove EB from the vessel. Subsequently, the brain was removed, and the hemispheres were separated as well. The brain tissue was homogenized in 2.5 mL phosphate buffer saline, and then 2.5 mL of trichloroacetic acid 60% (Merck, Germany) was added to precipitate the protein content. The mixture was then vortexed for 3 minutes, cooled for 30 minutes, and centrifuged for 30 minutes at 1000 × g. The supernatants were measured at 610 nm for the absorbance of EB using a spectrophotometer (UV-Visible, USA). The results were expressed as micrograms per gram of the brain tissue calculated according to a standard curve (15).
Real-Time Polymerase Chain Reaction
Total RNA was isolated from the infarcted areas of rats following MCAO and the corresponding regions of the brain of control rats by TRIzol Reagent Invitrogen (Sinacolon RN7713C). The cDNA was synthesized using the Takara Company’s PrimeScriptTM RT Reagent Kit (Perfect Real Time). Specific primers for rat MMP-2, MMP-9, and β-actin (housekeeping gene) were considered for this experiment (Table 1) by applying Takara SYBR Premix Ex Taq Master (Takara Bio, Inc., Shiga, Japan). Gene expression levels were quantified by Applied BiosystemsTM RT-PCR Instruments. The relative expression ratios of MMP-2, MMP-9, and β-actin were calculated as described by Pfaffl using the relative expression software tool (15). The cycling parameters for qPCR included 10 minutes at 95°C for initial denaturation, followed by 40 cycles of 30 seconds at 95°C, 30 seconds at optimum temperature for each gene, and 30 seconds at 72°C.
Table 1.
Primer Sequence for RT-qPCR
|
Genes
|
Primer Sequences
|
| MMP-2 |
Forward: 5'-CGATGTCTCCCCCAAAACAG-3'
Reverse: 5'-GCAGCCATAGAAAGTGTTCA-3' |
| MMP-9 |
Forward: 5'-GCAAACCCTGCGTATTTCCAT-3'
Reverse: 5'-CCATCTTTGGACCGATTGCTG-3' |
| β-actin |
Forward: 5'-GCTCTGGCTCCTAGCACCAT -3'
Reverse: 5'-GCCACCGATCCACACAGAGT-3' |
Note. RT-qPCR: Real-time quantitative polymerase chain reaction.
ELISA Method for the Evaluation of IL-6 and TNF-α
First, the standard curve with certain concentrations was made according to the kit instructions, and their absorption rate was measured by the ELISA reader at the wavelength of 450-630 nm. Then, tissue samples from the brain were prepared according to the kit instructions, and their absorption was examined by the device.
Statistical Analysis
Data were analyzed using SPSS software. All data are presented as the mean ± standard error of the mean (SEM). The results of RT-PCR, ELISA test, lipid profiles, the volume of stroke, and BBB permeability were assessed by analysis of variance (ANOVA) and Duncan post hoc test, and BBB permeability in two hemispheres was compared by independent t test. In addition, the results of neurological defects were analyzed by Kruskal-Wallis and Mann-Whitney U tests, and a P value of less than 0.05 was considered statistically significant.
Results
Effects of the Cinnamon Extract on the Serum Lipid Profile
The serum levels of cholesterol, triglyceride, and LDL in three treatment groups, including lovastatin, low-dose cinnamon extract (Cin130), and high-dose of cinnamon extract (Cin260), were significantly less than the three groups of hyperlipidemia that received an HFD without any intervention. Reductions in the level of cholesterol, triglyceride, and LDL in the positive control group (lovastatin) were similar to the control group. The serum levels of cholesterol and triglyceride in the treatment group with the Cin130 were significantly lower than those of the Cin260, and the mean serum LDL level in the treatment group with Cin260 was significantly lower than that of the treatment group with Cin130. Based on the results, the mean serum concentration of HDL in the lovastatin group was significantly higher than in the control group (Table 2).
Table 2.
The Effect of the Hydro-alcoholic Extract of Cinnamon (130 and 260 mg/d, Cin) Pretreatment for 6 Weeks on Serum Lipid Levels
|
Lipids (mg/dL)
|
Control (NG: 1)
|
Sham (NG: 2)
|
Model (NG: 3)
|
Vehicle (NG: 4)
|
Lovastatin (NG: 5)
|
Cin130 (NG: 6)
|
Cin260 (NG: 7)
|
Statistical Results
|
| Cholesterol |
52 ± 7 |
71.3 ± 8 |
79 ± 13 |
71.8 ± 6 |
60.5 ± 7* |
61 ± 5* |
66.5 ± 11* |
*
P < 0.05 vs. Model and vehicle |
| Triglyceride |
57.7 ± 12 |
97.8 ± 17 |
109 ± 20 |
99.7 ± 18 |
73.8 ± 12* |
79.7 ± 11* |
87.3 ± 22* |
*
P < 0.05 vs. Model and vehicle |
| LDL |
15.3 ± 3 |
22 ± 2 |
24.3 ± 4 |
22.7 ± 5 |
16.8 ± 3** |
20.8 ± 2*** |
20.2 ± 4*** |
**
P < 0.01 vs. Vehicle
***P < 0.001 vs. Model |
| HDL |
25.3 ± 4 |
35.7 ± 6 |
35.3 ± 4 |
33.7 ± 5 |
38 ± 6 |
37.7 ± 5 |
34.1 ± 6 |
|
Note. LSD: Least significant difference; SEM: Standard error of the mean; ANOVA: Analysis of variance; LDL: Low-density lipoprotein; HDL: High-density lipoprotein. The data are expressed as the mean ± SEM. ANOVA and LSD post hoc test, NG: Number of group. Statistical results have shown according to the number of groups (n = 6).
Effects of the Cinnamon Extract on Neurological Deficits
The comparison of median neurologic deficit scores (NDS) in the five groups showed that NDS in the three treatment groups significantly decreased compared to the two groups of hyperlipidemia. The NDS in the treatment group with the Cin260 was significantly lower than the Cin130 (Figure 2).
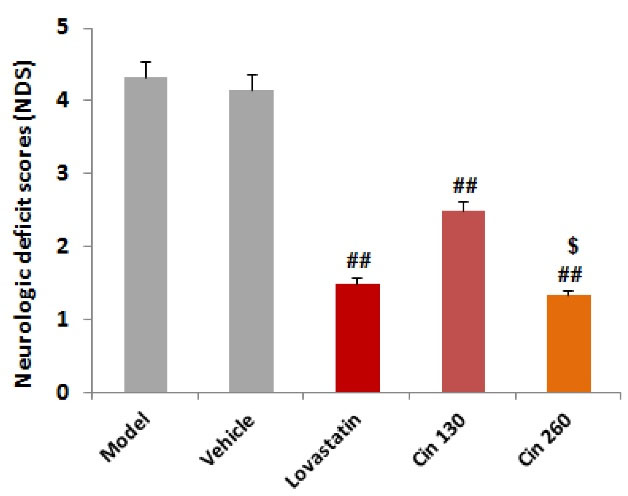
Figure 2.
The Distribution of Neurologic Deficit Score in Each Group. Note. Kruskal-Wallis and U-Mann Whitney tests. ## (P < 0.01) compared with the model and vehicle groups, $ (P < 0.05) compared with the Cin130 group (n = 6)
.
The Distribution of Neurologic Deficit Score in Each Group. Note. Kruskal-Wallis and U-Mann Whitney tests. ## (P < 0.01) compared with the model and vehicle groups, $ (P < 0.05) compared with the Cin130 group (n = 6)
Effects of the Cinnamon Extract on the Infarct Volume
The infarct volume in the core and penumbra areas underwent calculation. In the MCAO model group, 1 h-ischemia/12 h-reperfusion caused severe infarction in the cerebral cortex and subcortex areas. The infarct volume in the core and penumbra areas was measured in this study. Cinnamon extract pretreatment at doses of 130 and 260 mg/d for 4 weeks markedly reduced infarct volumes in the core and penumbra compared to the model group. The mean total volume of ischemia was significantly lower in the three treatment groups with lovastatin, Cin130, and Cin260 in comparison to model and vehicle groups. There was also a significant reduction in the amount of the core volume in the Cin260 group compared to the Cin130 group (Figure 3A & 3B).
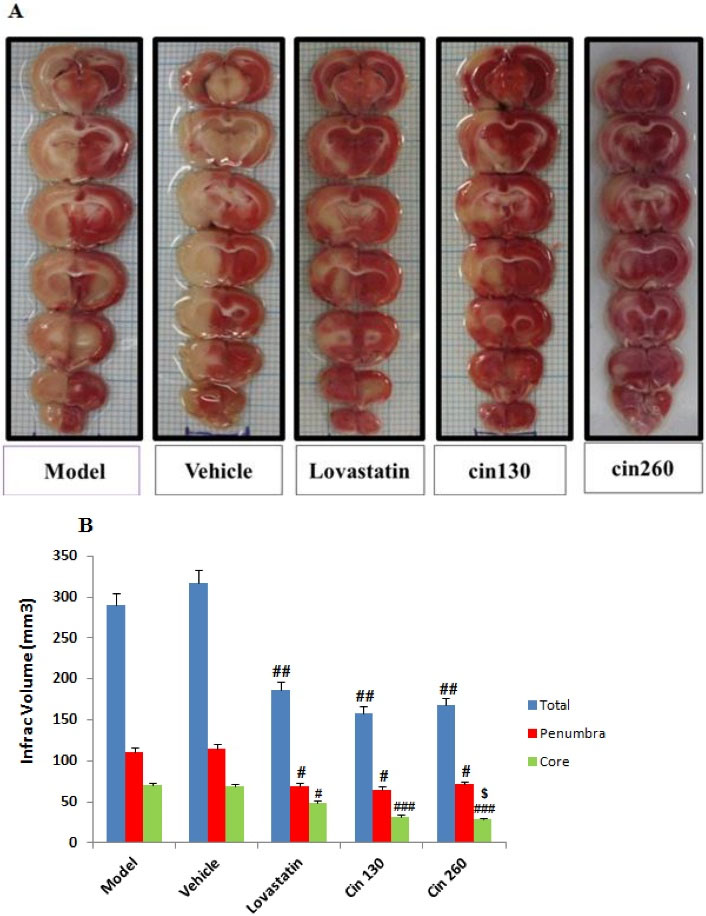
Figure 3.
Photograph (A) Shows the TTC-stained Brain Coronal Sections After Ischemia for 60 Minutes that Followed by Reperfusion for 12 Hours; White and Red Areas Represent Damaged and Surviving Regions, respectively; (B) Graph Displaying Brain Infarct Volumes in the Total Hemisphere in the MCAO Group Rats Pretreated With Cinnamon at the Doses of 130 and 260 mg/kg as Compared to the Corresponding Infarct Volume of Control MCAO Group Rats. Note. TTC: 2, 3, 5-Triphenyl-2-tetrazolium chloride; MCAO: Middle cerebral artery occlusion. The data are expressed as the mean ± SEM. (P < 0.01) compared with the control group,#(P < 0.05), ##(P < 0.01) and### (P < 0.001) compared with the model and vehicle groups, $ (P < 0.01) compared with the Cin130 group (n = 6)
.
Photograph (A) Shows the TTC-stained Brain Coronal Sections After Ischemia for 60 Minutes that Followed by Reperfusion for 12 Hours; White and Red Areas Represent Damaged and Surviving Regions, respectively; (B) Graph Displaying Brain Infarct Volumes in the Total Hemisphere in the MCAO Group Rats Pretreated With Cinnamon at the Doses of 130 and 260 mg/kg as Compared to the Corresponding Infarct Volume of Control MCAO Group Rats. Note. TTC: 2, 3, 5-Triphenyl-2-tetrazolium chloride; MCAO: Middle cerebral artery occlusion. The data are expressed as the mean ± SEM. (P < 0.01) compared with the control group,#(P < 0.05), ##(P < 0.01) and### (P < 0.001) compared with the model and vehicle groups, $ (P < 0.01) compared with the Cin130 group (n = 6)
Effects of the Cinnamon Extract on BBB
The BBB permeability to EB in the ischemic hemisphere in the three treatment groups was significantly less than that of the two groups receiving an HFD without any treatment intervention. The results represented significant differences in the permeability of the BBB between Cin130- and Cin260-treated groups. Conversely, there were no significant differences in the permeability of the BBB between the left hemispheres of the study groups (Figure 4).
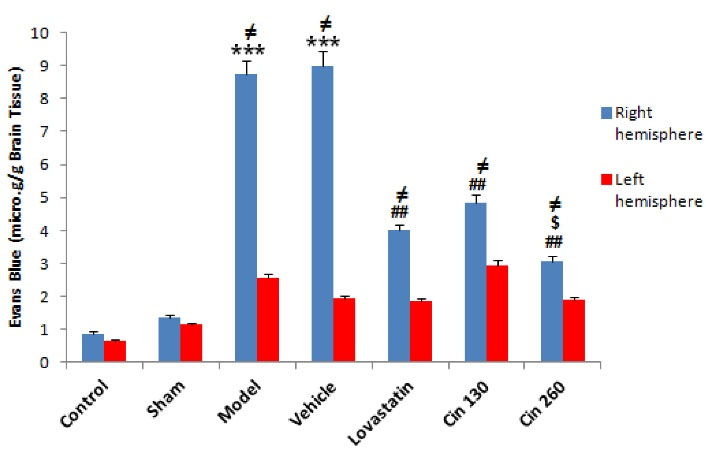
Figure 4.
Comparison of the Average Permeability of the BBB in the Damaged Hemisphere in Control, Sham, Model, Lovastatin, Vehicle, Low-dose Cinnamon (Cin 130) and High-dose Cinnamon (Cin 260) Groups. Note. Cin: Cinnamon; BBB: Blood-brain barrier; SEM: Standard error of the mean. The data are expressed as the mean ± SEM. ***(P < 0.001) compared with the control group, ## (P < 0.01) compared with the model and vehicle groups, $ (P < 0.01) compared with the Cin130 group, ≠ (P < 0.05) compared with the same left hemisphere (n = 6)
.
Comparison of the Average Permeability of the BBB in the Damaged Hemisphere in Control, Sham, Model, Lovastatin, Vehicle, Low-dose Cinnamon (Cin 130) and High-dose Cinnamon (Cin 260) Groups. Note. Cin: Cinnamon; BBB: Blood-brain barrier; SEM: Standard error of the mean. The data are expressed as the mean ± SEM. ***(P < 0.001) compared with the control group, ## (P < 0.01) compared with the model and vehicle groups, $ (P < 0.01) compared with the Cin130 group, ≠ (P < 0.05) compared with the same left hemisphere (n = 6)
Effects of the Cinnamon Extract on the Relative Expression of MMP-2,9
The results demonstrated that the relative expression of MMP2 (Figure 5A) and MMP9 (Figure 5B) genes was significantly increased in the stroke groups (model and vehicle) compared to the intact (control) group. However, the expression of these genes was significantly reduced in the three treated groups in comparison to the model and vehicle groups. Decreased expression of MMP genes was higher in the Cin260-treated group than in the Cin130-treated group.
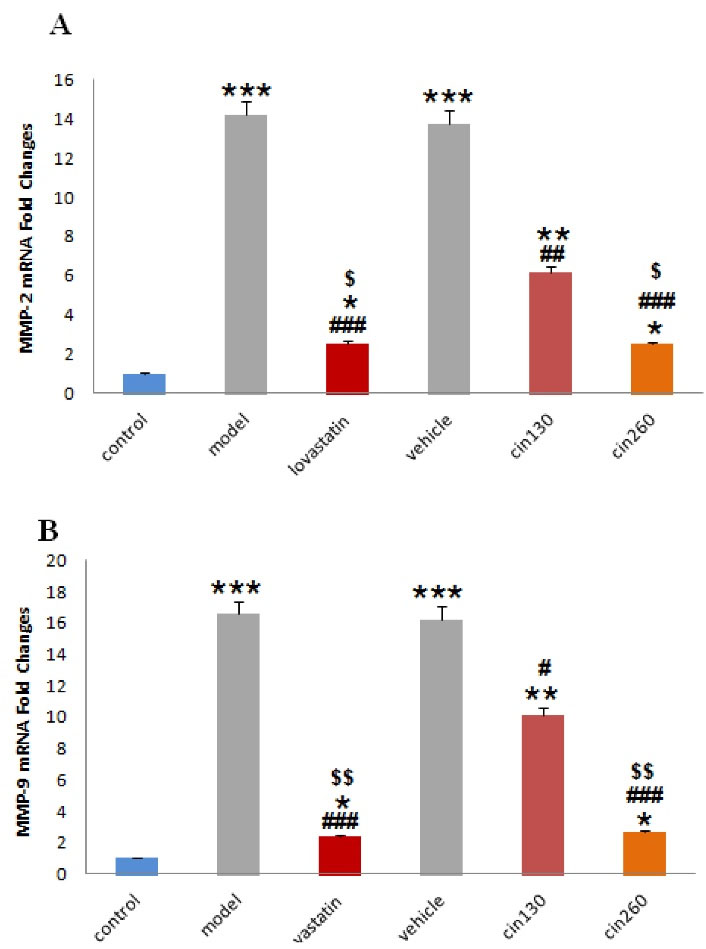
Figure 5.
Comparison of the Mean Relative Expression of MMP-2 (A) and MMP-9 (B) Gene in Control, Model, Lovastatin, Vehicle, and Low Dose Cinnamon (Cin130) and High-dose Cinnamon (Cin260) Groups. Note. MMP: Matrix metalloproteinase; Cin: Cinnamon. The data are expressed as the mean ± SEM *(P < 0.05), ** (P < 0.01) and *** (P < 0.001) compared with the control group, # (P < 0.05), ## (P < 0.01), and ###(P < 0.001) compared with the model and vehicle groups, $ (P < 0.05) and $$(P < 0.01) compared with the Cin130 group. Beta-actin was employed as the housekeeping gene (n = 6)
.
Comparison of the Mean Relative Expression of MMP-2 (A) and MMP-9 (B) Gene in Control, Model, Lovastatin, Vehicle, and Low Dose Cinnamon (Cin130) and High-dose Cinnamon (Cin260) Groups. Note. MMP: Matrix metalloproteinase; Cin: Cinnamon. The data are expressed as the mean ± SEM *(P < 0.05), ** (P < 0.01) and *** (P < 0.001) compared with the control group, # (P < 0.05), ## (P < 0.01), and ###(P < 0.001) compared with the model and vehicle groups, $ (P < 0.05) and $$(P < 0.01) compared with the Cin130 group. Beta-actin was employed as the housekeeping gene (n = 6)
Effect of Cinnamon on the Levels of IL-6 and TNF-α
Based on the results of the present study, cerebral ischemia and an HFD led to a significant increase in IL-6 (Figure 6A) and TNF-α (Figure 6B) levels. The use of both doses of the cinnamon extract significantly reduced the level of these factors in the rats of the treatment group compared to the model group, which was also observed in the group receiving lovastatin. The decrease in the level of these factors was greater in the group receiving the high dose of the extract in comparison to the groups receiving the low dose and lovastatin. The differences between the mentioned groups were significant.
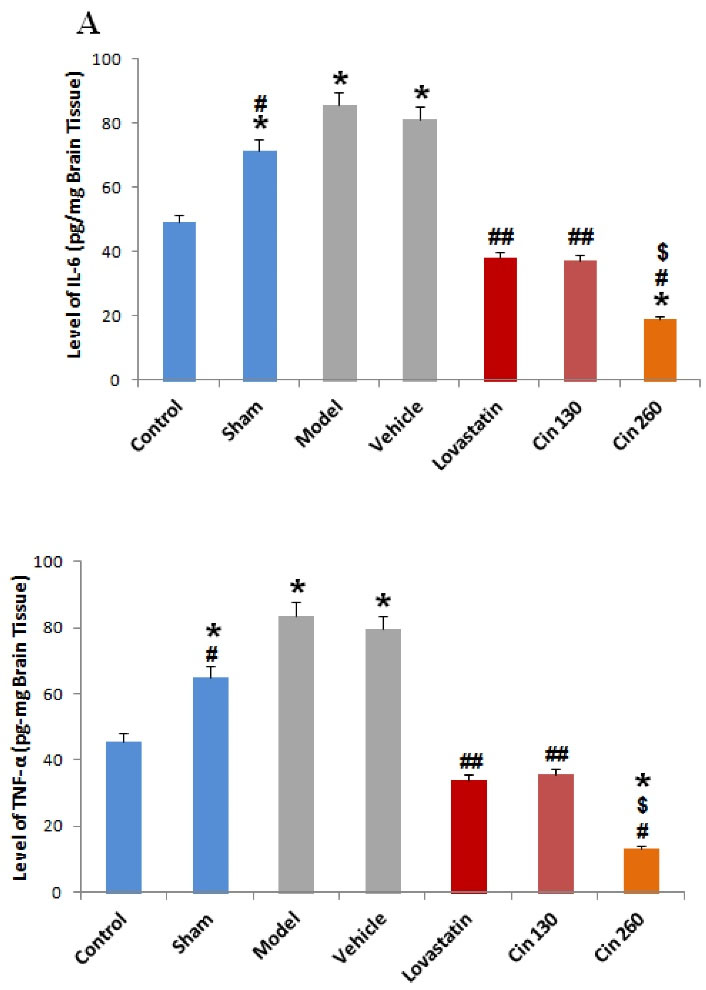
Figure 6.
Comparison of IL-6 (A) and TNF-α (B) Levels in Different Study Groups. Note. IL-6: Interleukin 6; TNF-α: Tumor necrosis factor α; SEM: Standard error of the mean; Cin: Cinnamon. The data are expressed as the mean ± S.E.M. *(P < 0.05) compared with the control group, #(P < 0.05), ## (P < 0.01) compared with the model and vehicle groups, $ (P < 0.01) compared with the Cin130 group. Beta-actin was used as the housekeeping gene (n = 6)
.
Comparison of IL-6 (A) and TNF-α (B) Levels in Different Study Groups. Note. IL-6: Interleukin 6; TNF-α: Tumor necrosis factor α; SEM: Standard error of the mean; Cin: Cinnamon. The data are expressed as the mean ± S.E.M. *(P < 0.05) compared with the control group, #(P < 0.05), ## (P < 0.01) compared with the model and vehicle groups, $ (P < 0.01) compared with the Cin130 group. Beta-actin was used as the housekeeping gene (n = 6)
Discussion
The present study investigated the effect of pre-treatment with the hydroalcoholic extract of cinnamon on variables related to stroke in hyperlipidemic rats. The results of this study revealed that the extract had neuropreventive effects. This extract had positive effects on the serum, tissue, and molecular variables, and it was found that part of the effects of cinnamon could be due to its effect on the MMPs and cytokines.
One of the main risk factors for stroke is hyperlipidemia (2). It has been shown that hyperlipidemia exacerbates the increase in MMP-2,9 expression levels in rats receiving an HFD and leads to further degradation of tight junction proteins, resulting in more severe BBB damages and ischemic complications. Moreover, the use of lipid-controlling agents leads to the down-regulation of MMP-2,9, strengthens the tight junction proteins, and improves BBB integrity (35,36). There is a direct relationship between hyperlipidemia and the severity of inflammation during cerebral ischemia, exacerbating damage to BBB (17). According to previous evidence, the increase in MMP expression levels and inflammatory cytokines following MCAO leads to the spread of lesions due to cerebral ischemia in rats, and the use of pretreatments that prevent the up-regulation of these factors strengthens the BBB integrity and mitigates the cerebral ischemia complications, including edema and neurological defects (15).
According to previous research, cinnamon plays a role in improving lipid profiles by the up-regulation of LDL receptor gene expression (36), inhibiting the 3-hydroxy-3-methyl-glutaryl coenzyme A reductase, decreasing lipid peroxidation by inhibiting 5-lipoxygenase, and increasing the amount of antioxidant enzymes (37,38). Studies on animal models have reported that cinnamon can prevent obesity caused by consuming an HFD and its associated complications such as hyperlipidemia and OS (39,40). In fact, cinnamon is effective in fat reduction through modulating a variety of transcription factors and enzymes, lipid metabolism, and the body’s antioxidant status (21). The pathophysiology of I/R leads to the production of inflammatory factors such as IL-6, IL-1β, and TNF-α, which can worsen the condition (12,15). On the other hand, many studies have focused on the anti-inflammatory properties of cinnamon as a medicinal plant (41). The findings of the present study demonstrated that cinnamon significantly reduces cholesterol, triglyceride, and LDL levels and thus leads to lipid profile control. Additionally, using different doses of this extract significantly reduces the level of IL-6 and TNF-α proteins, as well as MMP-2,9 expression levels. It also considerably decreases the amount of Evans blue in the affected hemisphere. Furthermore, neurological defects in rats receiving the extract were significantly reduced compared to the control group. Therefore, it seems that the cinnamon extract strengthens BBB and mitigates neurological defects by modulating the lipid profile and reducing the expression of inflammatory factors and metalloproteinases.
There were significant differences between Cin130 and Cin260 on BBB permeability. Further, MMP expression was lower in Cin260 compared with Cin130. According to previous studies, the disruption of the BBB in the course of ischemia/reperfusion injury occurs in two stages. The first stage is due to the redistribution of tight junction (TJ) and adherens junction (AJ) proteins from the membrane to the cytoplasm. The second stage is because of the degradation of TJ and basal lamina proteins by MMPs (42). The permeability of the BBB in this study was assessed 12 hours after ischemia induction, probably not providing the time required for the MMPs to function. It seems that the effects of MMPs could be observed if the time interval between the evaluation of BBB permeability and ischemia induction was longer.
Consuming an HFD can lead to obesity and its associated complications, including hyperlipidemia and OS (39,40). Hyperlipidemia exacerbates ischemic complications due to endothelial cell damage, OS, inflammation, and neuronal death. OS leads to increased production of free radicals and decreased antioxidant capacity, thereby intensifying damage to DNA and cell proteins. Additionally, it induces apoptosis through the activation of caspase 3 and 9 (43).
The findings of a study demonstrated that the expression of MMP9 genes in the animal model of ischemia (MCAO) and patients with stroke increases, leading to neuronal damage, apoptosis, and destruction of the BBB. It was further reported that inhibiting MMP9 gene expression is a possible solution and treatment to improve the condition in patients with stroke (44). It has also been shown that increased MMP9 production is one of the factors involved in neuronal death, and the suppression of this increased expression is one of the factors that can be associated with the functional recovery of neurons (45). Evidence also represents that TNF-α and increased production of free radicals can stimulate the release of MMP9, and increased MMP9 expression is closely related to increased P53 expression, which is involved in cell death (46). Therefore, it seems that increasing the body’s antioxidant capacity can be effective in controlling the consequences of hyperlipidemia during cerebral ischemia, including tissue damage due to neuronal death.
In recent years, the revelation of the antioxidative and neuroprotective characteristics of natural herbal products has drawn serious attention. It is noteworthy that several natural herbal products have been confirmed to keep neuronal cells from death in several studies (47,48). On the other hand, Jana et al stated that cinnamon and sodium benzoate present in it increased the level of neurotrophic factors such as BDNF (49). Moreover, phenolic compounds present in cinnamon, which can prevent the effects of free radicals, are likely to affect the results of this study because phenolic compounds have redox properties, which have an antioxidant effect (21,50). It seems that parts of the results in this study can be attributed to the flavonoid compounds of cinnamon. These compounds have potency in reducing inflammatory factors and damage caused by these factors. Lee et al found that the cinnamon extract inhibits TNF and COX-2, thereby inhibiting the production of prostaglandin E, and reducing inflammatory effects (51). Due to the presence of compounds such as cinnamaldehyde and flavonoids, cinnamon seems to be effective in reducing or suppressing inflammatory pathways and factors, thereby reducing the amount of damage caused by I/R (15,19). Regarding the effect of cinnamon on reducing the volume of stroke, it can be mentioned that by reducing or inhibiting the production of free radicals and its antioxidant properties, cinnamon can act to prevent lipid peroxidation and subsequently reduce the size of the ischemic region of the affected area (21,31).
Based on the above-mentioned observations, which confirm the antioxidant effects of cinnamon, this extract can be effective in reducing OS and tissue damage. There are also other mechanisms involved in reducing tissue damage, including the effect of this extract on reducing the level of MMP-2,9 and inflammatory cytokines, thus reducing neuronal damage. Thus, it seems that through several mechanisms, this extract can exert its protective effects during cerebral ischemia and reduce its complications in hyperlipidemic conditions.
In conclusion, the findings of this study suggest that cinnamon is effective as a preventive factor in reducing the volume of stroke, neurological defects, the permeability of the BBB, and the damaging effects of the MMPs in the acute phase of stroke. The mechanisms of these effects may be due, at least in part, to reduce the expression of these genes. Briefly, cinnamon, in addition to its lipid-lowering effect, may have a neuroprotective effect that may be related to the down-regulation of MMPs and cytokines. The two may be independent events or related to a third event, in any case, appropriate experiments should be performed in future studies to determine whether altering the MMPs reduces the effectiveness of cinnamon.
Acknowledgments
The authors would like to express their gratitude for the support (Grant #A-10-871-7) of the Deputy of Research, Zanjan University of Medical Sciences, Zanjan, Iran.
Authors’ Contributions
Conceptualization: Mahin Ghanjkhani, Seyyed Saeid Mousavi, Mojtaba Fathi, Mehdi Eskandari, Hossein Mostafavi.
Data curation: Mohammad Reza Mostajabi, Maryam Tavabi, Massoud Hatami.
Formal analysis: Hossein Mostafavi, Seyyed Saeid Mousavi.
Funding acquisition: Hossein Mostafavi.
Investigation: Mahin Ghanjkhani, Seyyed Saeid Mousavi, Mojtaba Fathi, Mehdi Eskandari, Hossein Mostafavi.
Methodology: Mohammad Reza Mostajabi, Maryam Tavabi, Massoud Hatami, Meysam Forouzandeh, Elham Ghasemloo.
Project administration: Hossein Mostafavi.
Resources: Mahin Ghanjkhani, Seyyed Saeid Mousavi, Mojtaba Fathi, Mehdi Eskandari, Hossein Mostafavi.
Software: Hossein Mostafavi, Seyyed Saeid Mousavi.
Supervision: Mahin Ghanjkhani, Seyyed Saeid Mousavi, Mojtaba Fathi, Mehdi Eskandari, Hossein Mostafavi.
Validation: Mahin Ghanjkhani, Seyyed Saeid Mousavi, Mojtaba Fathi, Mehdi Eskandari, Hossein Mostafavi.
Visualization: Mahin Ghanjkhani, Seyyed Saeid Mousavi, Mojtaba Fathi, Mehdi Eskandari, Hossein Mostafavi.
Writing – original draft: Mohammad Reza Mostajabi, Maryam Tavabi, Massoud Hatami.
Writing – review & editing: Meysam Forouzandeh, Elham Ghasemloo.
Competing Interests
The authors declare that they have no competing interests.
Ethical Approval
The animals were tested according to the guidelines of the International Organization for Medical Science Working with Laboratory Animals. The protocol for working with animals was approved by the Ethics Committee of Zanjan University of Medical Sciences (Ethical No: ZUMS.REC.1396.75).
Funding
This study was supported by grant #A-10-871-7 from the Deputy of Research, Zanjan University of Medical Sciences, Zanjan, Iran.
References
- Farhoudi M, Mehrvar K, Sadeghi-Bazargani H, Hashemilar M, Seyedi-Vafaee M, Sadeghi-Hokmabad E. Stroke subtypes, risk factors and mortality rate in northwest of Iran. Iran J Neurol 2017; 16(3):112-7. [ Google Scholar]
- George MG. Risk factors for ischemic stroke in younger adults: a focused update. Stroke 2020; 51(3):729-35. doi: 10.1161/strokeaha.119.024156 [Crossref] [ Google Scholar]
- Qawasmeh MA, Aldabbour B, Momani A, Obiedat D, Alhayek K, Kofahi R. Epidemiology, risk factors, and predictors of disability in a cohort of Jordanian patients with the first ischemic stroke. Stroke Res Treat 2020; 2020:1920583. doi: 10.1155/2020/1920583 [Crossref] [ Google Scholar]
- Wu Q, Cui J, Xie Y, Wang M, Zhang H, Hu X. Outcomes of ischemic stroke and associated factors among elderly patients with large-artery atherosclerosis: a hospital-based follow-up study in China. Front Neurol 2021; 12:642426. doi: 10.3389/fneur.2021.642426 [Crossref] [ Google Scholar]
- Grisotto C, Taïlé J, Planesse C, Diotel N, Gonthier MP, Meilhac O. High-fat diet aggravates cerebral infarct, hemorrhagic transformation and neuroinflammation in a mouse stroke model. Int J Mol Sci 2021; 22(9):4571. doi: 10.3390/ijms22094571 [Crossref] [ Google Scholar]
- Mostafa DG, Satti HH, Khaleel EF, Badi RM. A high-fat diet rich in corn oil exaggerates the infarct size and memory impairment in rats with cerebral ischemia and is associated with suppressing osteopontin and Akt, and activating GS3Kβ, iNOS, and NF-κB. J Physiol Biochem 2020; 76(3):393-406. doi: 10.1007/s13105-020-00744-2 [Crossref] [ Google Scholar]
- Hoane MR, Swan AA, Heck SE. The effects of a high-fat sucrose diet on functional outcome following cortical contusion injury in the rat. Behav Brain Res 2011; 223(1):119-24. doi: 10.1016/j.bbr.2011.04.028 [Crossref] [ Google Scholar]
- Eid RA, Eleawa SM, Alkhateeb MA, Aldera H, Zaki MSA, Al-Shraim M. Chronic consumption of a high-fat diet rich in corn oil activates intrinsic cell death pathway and induces several ultrastructural changes in the atria of healthy and type 1 diabetic rat. Clin Exp Pharmacol Physiol 2019; 46(12):1111-23. doi: 10.1111/1440-1681.13158 [Crossref] [ Google Scholar]
- Sun MS, Jin H, Sun X, Huang S, Zhang FL, Guo ZN. Free radical damage in ischemia-reperfusion injury: an obstacle in acute ischemic stroke after revascularization therapy. Oxid Med Cell Longev 2018; 2018:3804979. doi: 10.1155/2018/3804979 [Crossref] [ Google Scholar]
- Adibhatla RM, Hatcher JF. Phospholipase A2, reactive oxygen species, and lipid peroxidation in CNS pathologies. BMB Rep 2008; 41(8):560-7. doi: 10.5483/bmbrep.2008.41.8.560 [Crossref] [ Google Scholar]
- Rempe RG, Hartz AMS, Bauer B. Matrix metalloproteinases in the brain and blood-brain barrier: versatile breakers and makers. J Cereb Blood Flow Metab 2016; 36(9):1481-507. doi: 10.1177/0271678x16655551 [Crossref] [ Google Scholar]
- Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia/Reperfusion. Compr Physiol 2016; 7(1):113-70. doi: 10.1002/cphy.c160006 [Crossref] [ Google Scholar]
- Lewis A, Segal A. Hyperlipidemia and primary prevention of stroke: does risk factor identification and reduction really work?. Curr Atheroscler Rep 2010; 12(4):225-9. doi: 10.1007/s11883-010-0117-4 [Crossref] [ Google Scholar]
- Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care 2013; 40(1):195-211. doi: 10.1016/j.pop.2012.11.003 [Crossref] [ Google Scholar]
- Ghasemloo E, Oryan S, Bigdeli MR, Mostafavi H, Eskandari M. The neuroprotective effect of MicroRNA-149-5p and coenzymeQ10 by reducing levels of inflammatory cytokines and metalloproteinases following focal brain ischemia in rats. Brain Res Bull 2021; 169:205-13. doi: 10.1016/j.brainresbull.2021.01.013 [Crossref] [ Google Scholar]
- Ghasemloo E, Mostafavi H, Hosseini M, Forouzandeh M, Eskandari M, Mousavi SS. Neuroprotective effects of coenzyme Q10 in Parkinson's model via a novel Q10/miR-149-5p/MMPs pathway. Metab Brain Dis 2021; 36(7):2089-100. doi: 10.1007/s11011-021-00795-4 [Crossref] [ Google Scholar]
- Fang W, Sha L, Kodithuwakku ND, Wei J, Zhang R, Han D. Attenuated blood-brain barrier dysfunction by XQ-1H following ischemic stroke in hyperlipidemic rats. Mol Neurobiol 2015; 52(1):162-75. doi: 10.1007/s12035-014-8851-1 [Crossref] [ Google Scholar]
- Sedighi M, Bahmani M, Asgary S, Beyranvand F, Rafieian-Kopaei M. A review of plant-based compounds and medicinal plants effective on atherosclerosis. J Res Med Sci 2017; 22:30. doi: 10.4103/1735-1995.202151 [Crossref] [ Google Scholar]
- Rao PV, Gan SH. Cinnamon: a multifaceted medicinal plant. Evid Based Complement Alternat Med 2014; 2014:642942. doi: 10.1155/2014/642942 [Crossref] [ Google Scholar]
- Nayak IN, Chinta R, Jetti R. Anti-atherosclerotic potential of aqueous extract of Cinnamomum zeylanicum bark against glucocorticoid induced atherosclerosis in Wistar rats. J Clin Diagn Res 2017; 11(5):FC19-FC23. doi: 10.7860/jcdr/2017/23910.9864 [Crossref] [ Google Scholar]
- Tuzcu Z, Orhan C, Sahin N, Juturu V, Sahin K. Cinnamon polyphenol extract inhibits hyperlipidemia and inflammation by modulation of transcription factors in high-fat diet-fed rats. Oxid Med Cell Longev 2017; 2017:1583098. doi: 10.1155/2017/1583098 [Crossref] [ Google Scholar]
- Ghosh T, Basu A, Adhikari D, Roy D, Pal AK. Antioxidant activity and structural features of Cinnamomum zeylanicum. 3 Biotech 2015; 5(6):939-47. doi: 10.1007/s13205-015-0296-3 [Crossref] [ Google Scholar]
- Stavinoha RC, Vattem DA. Potential neuroprotective effects of cinnamon. Int J Appl Res Nat Prod 2015; 8(3):24-46. [ Google Scholar]
- Wang J, Wang S, Yang J, Henning SM, Ezzat-Zadeh Z, Woo SL. Acute effects of cinnamon spice on post-prandial glucose and insulin in normal weight and overweight/obese subjects: a pilot study. Front Nutr 2020; 7:619782. doi: 10.3389/fnut.2020.619782 [Crossref] [ Google Scholar]
- Abdanipour A, Deheshjo F, Sohrabi D, Jafari Anarkooli I, Nejatbakhsh R. Neuroprotective effect of lovastatin through down-regulation of pro-apoptotic Mst1 gene expression in rat model pilocarpine epilepsy. Neurol Res 2018; 40(10):874-82. doi: 10.1080/01616412.2018.1497252 [Crossref] [ Google Scholar]
- Elkind MS, Flint AC, Sciacca RR, Sacco RL. Lipid-lowering agent use at ischemic stroke onset is associated with decreased mortality. Neurology 2005; 65(2):253-8. doi: 10.1212/01.wnl.0000171746.63844.6a [Crossref] [ Google Scholar]
- Marques C, Meireles M, Norberto S, Leite J, Freitas J, Pestana D. High-fat diet-induced obesity rat model: a comparison between Wistar and Sprague-Dawley rat. Adipocyte 2016; 5(1):11-21. doi: 10.1080/21623945.2015.1061723 [Crossref] [ Google Scholar]
- Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Schölmerich J. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol 2006; 36(3):485-501. doi: 10.1677/jme.1.01909 [Crossref] [ Google Scholar]
- Jadhav SB, Narayana Murthy PS, Singh MM, Jain GK. Distribution of lovastatin to bone and its effect on bone turnover in rats. J Pharm Pharmacol 2006; 58(11):1451-8. doi: 10.1211/jpp.58.11.0005 [Crossref] [ Google Scholar]
- Mirhadi K. Effect of intraperitoneally injection of different doses of lovastatin on pain and inflammatory response induced by formalin in mice. Am J Anim Vet Sci 2011; 6(4):160-5. [ Google Scholar]
- Ranasinghe P, Pigera S, Premakumara GA, Galappaththy P, Constantine GR, Katulanda P. Medicinal properties of 'true' cinnamon (Cinnamomum zeylanicum): a systematic review. BMC Complement Altern Med 2013; 13:275. doi: 10.1186/1472-6882-13-275 [Crossref] [ Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989; 20(1):84-91. doi: 10.1161/01.str.20.1.84 [Crossref] [ Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 1986; 17(3):472-6. doi: 10.1161/01.str.17.3.472 [Crossref] [ Google Scholar]
- Liu F, McCullough LD. Middle cerebral artery occlusion model in rodents: methods and potential pitfalls. J Biomed Biotechnol 2011; 2011:464701. doi: 10.1155/2011/464701 [Crossref] [ Google Scholar]
- ElAli A, Doeppner TR, Zechariah A, Hermann DM. Increased blood-brain barrier permeability and brain edema after focal cerebral ischemia induced by hyperlipidemia: role of lipid peroxidation and calpain-1/2, matrix metalloproteinase-2/9, and RhoA overactivation. Stroke 2011; 42(11):3238-44. doi: 10.1161/strokeaha.111.615559 [Crossref] [ Google Scholar]
- Kassaee SM, Goodarzi MT, Hayati Roodbari N, Yaghmaei P. The effects of Cinnamomum zeylanicum on lipid profiles and histology via up-regulation of LDL receptor gene expression in hamsters fed a high cholesterol diet. Jundishapur J Nat Pharm Prod 2017; 12(3):e37340. doi: 10.5812/jjnpp.37340 [Crossref] [ Google Scholar]
- Baker WL, Gutierrez-Williams G, White CM, Kluger J, Coleman CI. Effect of cinnamon on glucose control and lipid parameters. Diabetes Care 2008; 31(1):41-3. doi: 10.2337/dc07-1711 [Crossref] [ Google Scholar]
- Rahman S, Begum H, Rahman Z, Ara F, Iqbal MJ, Yousuf AK. Effect of cinnamon (Cinnamomum cassia) as a lipid lowering agent on hypercholesterolemic rats. J Enam Med Coll 2013; 3(2):94-8. [ Google Scholar]
- Li W, Shi YH, Yang RL, Cui J, Xiao Y, Wang B. Effect of somatostatin analog on high-fat diet-induced metabolic syndrome: involvement of reactive oxygen species. Peptides 2010; 31(4):625-9. doi: 10.1016/j.peptides.2009.11.008 [Crossref] [ Google Scholar]
- Shearn CT, Mercer KE, Orlicky DJ, Hennings L, Smathers-McCullough RL, Stiles BL. Short term feeding of a high fat diet exerts an additive effect on hepatocellular damage and steatosis in liver-specific PTEN knockout mice. PLoS One 2014; 9(5):e96553. doi: 10.1371/journal.pone.0096553 [Crossref] [ Google Scholar]
- Han X, Parker TL. Antiinflammatory activity of cinnamon (Cinnamomum zeylanicum) bark essential oil in a human skin disease model. Phytother Res 2017; 31(7):1034-8. doi: 10.1002/ptr.5822 [Crossref] [ Google Scholar]
- Mun-Bryce S, Rosenberg GA. Matrix metalloproteinases in cerebrovascular disease. J Cereb Blood Flow Metab 1998; 18(11):1163-72. doi: 10.1097/00004647-199811000-00001 [Crossref] [ Google Scholar]
- Cao XL, Du J, Zhang Y, Yan JT, Hu XM. Hyperlipidemia exacerbates cerebral injury through oxidative stress, inflammation and neuronal apoptosis in MCAO/reperfusion rats. Exp Brain Res 2015; 233(10):2753-65. doi: 10.1007/s00221-015-4269-x [Crossref] [ Google Scholar]
- Chaturvedi M, Kaczmarek L. Mmp-9 inhibition: a therapeutic strategy in ischemic stroke. Mol Neurobiol 2014; 49(1):563-73. doi: 10.1007/s12035-013-8538-z [Crossref] [ Google Scholar]
- Qing-Feng S, Ying-Peng X, Tian-Tong X. Matrix metalloproteinase-9 and p53 involved in chronic fluorosis induced blood-brain barrier damage and neurocyte changes. Arch Med Sci 2019; 15(2):457-66. doi: 10.5114/aoms.2019.83294 [Crossref] [ Google Scholar]
- Hong S, Park KK, Magae J, Ando K, Lee TS, Kwon TK. Ascochlorin inhibits matrix metalloproteinase-9 expression by suppressing activator protein-1-mediated gene expression through the ERK1/2 signaling pathway: inhibitory effects of ascochlorin on the invasion of renal carcinoma cells. J Biol Chem 2005; 280(26):25202-9. doi: 10.1074/jbc.M413985200 [Crossref] [ Google Scholar]
- Iriti M, Vitalini S, Fico G, Faoro F. Neuroprotective herbs and foods from different traditional medicines and diets. Molecules 2010; 15(5):3517-55. doi: 10.3390/molecules15053517 [Crossref] [ Google Scholar]
- Liu Y, Wang S, Kan J, Zhang J, Zhou L, Huang Y. Chinese herbal medicine interventions in neurological disorder therapeutics by regulating glutamate signaling. Curr Neuropharmacol 2020; 18(4):260-76. doi: 10.2174/1570159x17666191101125530 [Crossref] [ Google Scholar]
- Jana A, Modi KK, Roy A, Anderson JA, van Breemen RB, Pahan K. Up-regulation of neurotrophic factors by cinnamon and its metabolite sodium benzoate: therapeutic implications for neurodegenerative disorders. J Neuroimmune Pharmacol 2013; 8(3):739-55. doi: 10.1007/s11481-013-9447-7 [Crossref] [ Google Scholar]
- Alizadeh Behbahani B, Falah F, Lavi Arab F, Vasiee M, Tabatabaee Yazdi F. Chemical composition and antioxidant, antimicrobial, and antiproliferative activities of Cinnamomum zeylanicum bark essential oil. Evid Based Complement Alternat Med 2020; 2020:5190603. doi: 10.1155/2020/5190603 [Crossref] [ Google Scholar]
- Lee SH, Lee SY, Son DJ, Lee H, Yoo HS, Song S. Inhibitory effect of 2'-hydroxycinnamaldehyde on nitric oxide production through inhibition of NF-kappa B activation in RAW 2647 cells. Biochem Pharmacol 2005; 69(5):791-9. doi: 10.1016/j.bcp.2004.11.013 [Crossref] [ Google Scholar]